Furaneol
From Wikipedia, the free encyclopedia
| Furaneol[1] | |
|---|---|
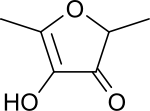 | |
| IUPAC name 4-Hydroxy-2,5-dimethyl-3-furanone | |
| Other names • 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | |
| Identifiers | |
| Abbreviations | DMHF |
| CAS number | 3658-77-3 |
| PubChem | 19309 |
| ChemSpider | 18218 |
| UNII | 20PI8YZP7A |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H8O3 |
| Molar mass | 128.13 g/mol |
| Melting point | 73–77 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Furaneol, or strawberry furanone, is a natural organic compound used in the flavor and perfume industry because of its sweet strawberry aroma.[2] It is actually found in strawberries[3] and a variety of other fruits and it is partly responsible for the smell of fresh pineapple.[4] It is also important for odour of buckwheat,[5] and tomato.[6]
References
- ↑ 4-Hydroxy-2,5-dimethyl-3(2H)-furanone at Sigma-Aldrich
- ↑ Strawberry furanone at thegoodscentscompany.com
- ↑ Ulrich, D. et al. 1995. Analysis of strawberry flavour - Quantification of the volatile components of varieties of cultivated and wild strawberries. Z. Lebensm. UNters. Forsch. 200:217-220
- ↑ Tokitomo Y, Steinhaus M, Büttner A, Schieberle P (2005). "Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation". Biosci. Biotechnol. Biochem. 69 (7): 1323–30. doi:10.1271/bbb.69.1323. PMID 16041138.
- ↑ Janes D, Kantar D, Kreft S, Prosen H (2008). "Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC-MS". Food Chemistry 112: 120. doi:10.1016/j.foodchem.2008.05.048.
- ↑ Buttery, R.G. et al. 2001. Analysis of furaneol in tomato using dynamic headspace sampling with sodium sulfate. J. Agric. Food Chem. 49:4349-4351
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.