Frenkel defect
The Frenkel defect is a type of defect shown by crystalline solids. It consists in the displacement of an atom from its lattice position to an interstitial site, creating a vacancy at the original site and an interstitial defect at the new location.
Definition
A Frenkel defect, Frenkel pair, or Frenkel disorder is a type of point defect in a crystal lattice. The defect forms when an atom or cation leaves its place in the lattice, creating a vacancy, and becomes an interstitial by lodging in a nearby location not usually occupied by an atom. Their prime mechanism of generation is by particle irradiation, as their equilibrium concentration according to the Boltzmann distribution is much smaller than the pure vacancies distribution, due to the large energy necessary for the creation of the associated interstitial atoms. The phenomenon is named after the Soviet physicist Yakov Frenkel, who discovered it in 1926.
Effect on Density
This defect does not appreciably change the density of the solid as it involves only the migration of the ions within the crystal, thus preserving both the volume as well as mass.
Examples
It is shown in ionic solids with large size difference between the anion and cation (with the cation usually smaller due to an increased effective nuclear charge) Some solids which display this defect - ZnS, AgCl, AgBr, AgI (due to the comparatively smaller size of Zn2+ and Ag+ ions)
To be noted : AgBr shows both Frenkel as well as Schottky defects.
For example, consider a lattice formed by X and M ions. Suppose an M ion leaves the M sublattice, leaving the X sublattice unchanged. The number of interstitials formed will equal the number of vacancies formed.
One form of a Frenkel defect reaction in MgO with the oxygen ion leaving the lattice and going into the interstitial site written in Kröger–Vink notation:
 +
+ →
→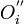 +
+ +
+
This can be illustrated with the example of the sodium chloride crystal structure. The diagrams below are schematic two-dimensional representations.


See also
- DLTS
- Schottky defect
- Wigner effect
- Crystallographic defects
References
- Kittel, Charles, Introduction to Solid State Physics - 8th ed. Wiley, 2005. ISBN 0-471-41526-X.