FourU thermometer
| FourU | |
|---|---|
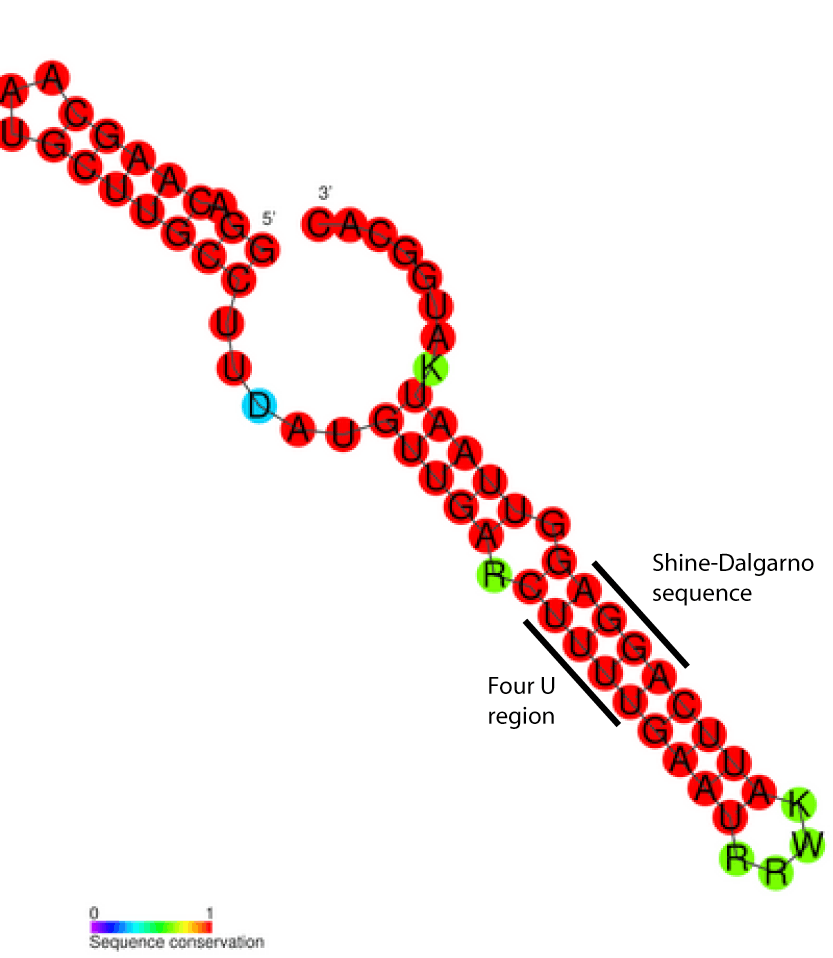 | |
| Consensus secondary structure of FourU RNA thermometers. Red colouring indicates the highest levels of nucleotide conservation. | |
| Identifiers | |
| Symbol | FourU |
| Rfam | RF01795 |
| Other data | |
| RNA type | RNA thermometer |
| Domain(s) | Salmonella; |
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella.[1] They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes.[2][3][4] FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.[1]
FourU are found in the 5' untranslated region of the gene for heat shock protein Salmonella agsA,[1][5][6] they repress translation of this protein by base-pairing the Shine-Dalgardo sequence of the gene's mRNA.[2] This prevents ribosomes from binding the gene's start codon.[7]
Other known RNA thermometers include the ROSE element[8][9] and Hsp90 cis-reg element.[10]
Response to temperature
Hairpin II appears to be a dynamic feature of FourU's secondary structure.[1][2] It undergoes a conformational shift when exposed to temperatures above 45°C, becoming increasingly unpaired as temperature rises.[1] Hairpin I, in contrast, remains stably base-paired in temperatures as high as 50°C, which implies the structural shift of hairpin II from closed to open may have an important role in heat shock response.[1] A later study used mutant analysis and calculations of enthalpy and entropy to support a cooperative zipper-type unfolding mechanism of FourU hairpin II in response to temperature increase.[2]
Sigma factor cooperation
Like other RNA thermometers, FourU is not solely responsible for temperature-dependent expression of its adjacent gene.[11] Instead, it operates in conjunction with a sigma factor (σ32)[12] which is known to also regulate many other genes.[13] Sigma factor-RNA thermometer combinations have been found to regulate other heat-shock genes (such as ibpA in E. coli)[4] which has led to speculation of undiscovered RNA thermometers operating alongside sigma factor modules to regulate other related genes as an additional level of control. Further speculation suggests the simpler RNA thermometer method of gene regulation may have evolved prior to the more complex sigma factor transcription control.[1]
agsA function
The agsA gene, which is regulated by FourU thermometers, was first discovered in Salmonella enterica.[6] The protein coded for by this gene is a small heat shock protein (sHSP) which protects bacteria from irreversible aggregation of proteins and aids in their refolding.[12] Mutant analysis confirmed the importance of agsA: a plasmid containing the gene and a promoter increased the survival rate of a thermosenstive mutant phenotype by remedying protein aggregation at high temperatures.[6] It has a similar function to the human chaperone α-crystallin.[14]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Waldminghaus, Torsten; Heidrich, Nadja; Brantl, Sabine; Narberhaus, Franz (2007). "FourU: A novel type of RNA thermometer in Salmonella". Molecular Microbiology 65 (2): 413–24. doi:10.1111/j.1365-2958.2007.05794.x. PMID 17630972.
- ↑ 2.0 2.1 2.2 2.3 Rinnenthal, J.; Klinkert, B.; Narberhaus, F.; Schwalbe, H. (2010). "Direct observation of the temperature-induced melting process of the Salmonella fourU RNA thermometer at base-pair resolution". Nucleic Acids Research 38 (11): 3834–47. doi:10.1093/nar/gkq124. PMC 2887971. PMID 20211842.
- ↑ Narberhaus, Franz; Waldminghaus, Torsten; Chowdhury, Saheli (2006). "RNA thermometers". FEMS Microbiology Reviews 30 (1): 3–16. doi:10.1111/j.1574-6976.2005.004.x. PMID 16438677.
- ↑ 4.0 4.1 Waldminghaus, Torsten; Fippinger, Anja; Alfsmann, Juliane; Narberhaus, Franz (2005). "RNA thermometers are common in α- and γ-proteobacteria". Biological Chemistry 386 (12): 1279–86. doi:10.1515/BC.2005.145. PMID 16336122.
- ↑ "aggregation suppressing protein". National Center for Biotechnology Information.
- ↑ 6.0 6.1 6.2 Tomoyasu, T.; Takaya, A.; Sasaki, T.; Nagase, T.; Kikuno, R.; Morioka, M.; Yamamoto, T. (2003). "A New Heat Shock Gene, agsA, Which Encodes a Small Chaperone Involved in Suppressing Protein Aggregation in Salmonella enterica Serovar Typhimurium". Journal of Bacteriology 185 (21): 6331–9. doi:10.1128/JB.185.21.6331-6339.2003. PMC 219406. PMID 14563868.
- ↑ Shine, J.; Dalgarno, L. (1975). "Determinant of cistron specificity in bacterial ribosomes". Nature 254 (5495): 34–8. doi:10.1038/254034a0. PMID 803646.
- ↑ Nocker, A.; Hausherr, T; Balsiger, S; Krstulovic, NP; Hennecke, H; Narberhaus, F (2001). "A mRNA-based thermosensor controls expression of rhizobial heat shock genes". Nucleic Acids Research 29 (23): 4800–7. doi:10.1093/nar/29.23.4800. PMC 96696. PMID 11726689.
- ↑ Waldminghaus, Torsten; Gaubig, Lena C.; Narberhaus, Franz (2007). "Genome-wide bioinformatic prediction and experimental evaluation of potential RNA thermometers". Molecular Genetics and Genomics 278 (5): 555–64. doi:10.1007/s00438-007-0272-7. PMID 17647020.
- ↑ Ahmed, R.; Duncan, RF (2004). "Translational Regulation of Hsp90 mRNA: AUG-PROXIMAL 5'-UNTRANSLATED REGION ELEMENTS ESSENTIAL FOR PREFERENTIAL HEAT SHOCK TRANSLATION". Journal of Biological Chemistry 279 (48): 49919–30. doi:10.1074/jbc.M404681200. PMID 15347681.
- ↑ Johansson, JöRgen; Mandin, Pierre; Renzoni, Adriana; Chiaruttini, Claude; Springer, Mathias; Cossart, Pascale (2002). "An RNA Thermosensor Controls Expression of Virulence Genes in Listeria monocytogenes". Cell 110 (5): 551–61. doi:10.1016/S0092-8674(02)00905-4. PMID 12230973.
- ↑ 12.0 12.1 Bukau, Bernd (1993). "Regulation of the Escherichia coli heat-shock response". Molecular Microbiology 9 (4): 671–80. doi:10.1111/j.1365-2958.1993.tb01727.x. PMID 7901731.
- ↑ Permina, E.A.; Gelfand, M.S. (2003). "Heat Shock (σ32 and HrcA/CIRCE) Regulons in β-, γ- and ε-Proteobacteria". Journal of Molecular Microbiology and Biotechnology 6 (3–4): 174–81. doi:10.1159/000077248. PMID 15153770.
- ↑ Rajaraman, K; Raman, B; Ramakrishna, T; Rao, CM (2001). "Interaction of human recombinant αA- and αB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation". FEBS Letters 497 (2–3): 118–23. doi:10.1016/S0014-5793(01)02451-6. PMID 11377425.
Further reading
- Vogel, Jörg (2009). "A rough guide to the non-coding RNA world ofSalmonella". Molecular Microbiology 71 (1): 1–11. doi:10.1111/j.1365-2958.2008.06505.x. PMID 19007416.
- Jin, Haining; Zhao, Qing; Gonzalez De Valdivia, Ernesto I.; Ardell, David H.; Stenstrom, Magnus; Isaksson, Leif A. (2006). "Influences on gene expression in vivo by a Shine-Dalgarno sequence". Molecular Microbiology 60 (2): 480–92. doi:10.1111/j.1365-2958.2006.05110.x. PMID 16573696.
- Chowdhury, S.; Ragaz, C; Kreuger, E; Narberhaus, F (2003). "Temperature-controlled Structural Alterations of an RNA Thermometer". Journal of Biological Chemistry 278 (48): 47915–21. doi:10.1074/jbc.M306874200. PMID 12963744.
- Kaempfer, Raymond (2003). "RNA sensors: Novel regulators of gene expression". EMBO Reports 4 (11): 1043–7. doi:10.1038/sj.embor.embor7400005. PMC 1326375. PMID 14593443.