Forward osmosis

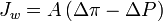
where 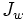 is water flux, A is the hydraulic permeability of the membrane, Δπ is the difference in osmotic pressures on the two sides of the membrane, and ΔP is the difference in hydrostatic pressure (negative values of
is water flux, A is the hydraulic permeability of the membrane, Δπ is the difference in osmotic pressures on the two sides of the membrane, and ΔP is the difference in hydrostatic pressure (negative values of 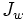 indicating reverse osmotic flow). The modeling of these relationships is in practice more complex than this equation indicates, with flux depending on the membrane, feed, and draw solution characteristics, as well as the fluid dynamics within the process itself.[1]
indicating reverse osmotic flow). The modeling of these relationships is in practice more complex than this equation indicates, with flux depending on the membrane, feed, and draw solution characteristics, as well as the fluid dynamics within the process itself.[1]
An additional distinction between the reverse osmosis (RO) and forward osmosis (FO) processes is that the water permeating the RO process is in most cases fresh water ready for use. In the FO process, this is not the case. The membrane separation of the FO process in effect results in a "trade" between the solutes of the feed solution and the draw solution. Depending on the concentration of solutes in the feed (which dictates the necessary concentration of solutes in the draw) and the intended use of the product of the FO process, this step may be all that is required.
The forward osmosis process is also known as osmosis or in the case of a number of companies who have coined their own terminology 'engineered osmosis' and 'manipulated osmosis'.
Applications
Emergency drinks

One example of an application of this type may be found in "hydration bags", which use an ingestible draw solute and are intended for separation of water from dilute feeds. This allows, for example, the ingestion of water from surface waters (streams, ponds, puddles, etc.) that may be expected to contain pathogens or toxins that are readily rejected by the FO membrane. With sufficient contact time, such water will permeate the membrane bag into the draw solution, leaving the undesirable feed constituents behind. The diluted draw solution may then be ingested directly. Typically, the draw solutes are sugars such as glucose or fructose, which provide the additional benefit of nutrition to the user of the FO device. A point of additional interest with such bags is that they may be readily used to recycle urine, greatly extending the ability of a backpacker or soldier to survive in arid environments.[2] This process may also, in principle, be employed with highly concentrated saline feedwater sources such as seawater, as one of the first intended uses of FO with ingestible solutes was for survival in life rafts at sea.[3]
Desalination

Desalinated water can be produced from the diluted draw / osmotic agent solution, using a second process. This may be by membrane separation, thermal method, physical separation or a combination of these processes. The process has the feature of inherently low fouling because of the forward osmosis first step, unlike conventional reverse osmosis desalination plants where fouling is often a problem. Modern Water plc has deployed forward osmosis based desalination plants in Gibraltar and Oman.[4][5][6] In March 2010, National Geographic[7] magazine cited forward osmosis as one of three technologies that promised to reduce the energy requirements of desalination.
Evaporative cooling tower – make-up water
One other application developed, where only the forward osmosis step is used, is in evaporative cooling make-up water. In this case the cooling water is the draw solution and the water lost by evaporation is simply replaced using water produced by forward osmosis from a suitable source, such as seawater, brackish water, treated sewage effluent or industrial waste water. Thus in comparison with other ‘desalination’ processes that may be used for make-up water the energy consumption is a fraction of these with the added advantage of the low fouling propensity of a forward osmosis process.[8][9][10]
Landfill leachate treatment
In the case where the desired product is fresh water which does not contain draw solutes, a second separation step is required. The first separation step of FO, driven by an osmotic pressure gradient, does not require a significant energy input (only unpressurized stirring or pumping of the solutions involved). The second separation step, however does typically require energy input. One method used for the second separation step is to employ RO. This approach has been used, for instance, in the treatment of landfill leachate. A FO membrane separation is used to draw water from the leachate feed into a saline (NaCl) brine. The diluted brine is then passed through a RO process to produce fresh water and a reusable brine concentrate. The advantage of this method is not a savings in energy, but rather in the fact that the FO process is more resistant to fouling from the leachate feed than a RO process alone would be.[11] A similar FO/RO hybrid has been used for the concentration of food products, such as fruit juice.[12]
Brine concentration
Brine concentration using forward osmosis can be achieved using a high molarity draw solution and high rejection membrane to produce fresh water. The current process uses the ammonia-carbon dioxide forward osmosis process originally developed at Yale University[13] and commercialized by Oasys Water. Because ammonia and carbon dioxide readily dissociate into gases using heat, the draw solutes can effectively be recovered and reused in a closed loop system. Brine concentration is currently being used in the oil and gas industry to treat produced water in the Permian Basin area of Texas.[14]
Research
An area of current research in FO involves direct removal of draw solutes, in this case by means of a magnetic field. Small (nanoscale) magnetic particles are suspended in solution creating osmotic pressures sufficient for the separation of water from a dilute feed. Once the draw solution containing these particles has been diluted by the FO water flux, they may be separated from that solution by use of a magnet (either against the side of a hydration bag, or around a pipe in-line in a steady state process).
References
- ↑ Lee, K (1981). "Membranes for power-generation by pressure-retarded osmosis". Journal of Membrane Science 8 (2): 141–171. doi:10.1016/S0376-7388(00)82088-8.
- ↑ Salter, R.J. (2005). "Forward Osmosis". Water Conditioning and Purification 48 (4): 36–38.
- ↑ Kessler, J.O.; Moody, C.D. (1976). "Drinking water from sea water by forward osmosis". Desalination 18 (3): 297–306. doi:10.1016/S0011-9164(00)84119-3.
- ↑ "FO plant completes 1-year of operation". Water Desalination Report: 2–3. 15 Nov. 2010. Retrieved 28 May 2011.
- ↑ "Modern Water taps demand in Middle East". The Independent. 23 Nov. 2009.
- ↑ Thompson N.A., Nicoll P.G. (September 2011). "Forward Osmosis Desalination: A Commercial Reality". Proceedings of the IDA World Congress. Perth, Western Australia: International Desalination Association.
- ↑ "The Big Idea". National Geographic. March 2010. Retrieved 14 June 2013.
- ↑ P. Nicoll Manipulated Osmosis – an alternative to Reverse Osmosis? Climate Control Middle East, April 2011, 46–49
- ↑ Nicoll P.G., Thompson N.A., Bedford M.R. (September 2011). "Manipulated Osmosis Applied To Evaporative Cooling Make-Up Water – Revolutionary Technology". Proceedings of the IDA World Congress. Perth, Western Australia: International Desalination Association.
- ↑ Peter Nicoll, Neil Thompson, Victoria Gray (February 2012). "Forward Osmosis Applied to Evaporative Cooling Make-up Water". Cooling Technology Institute.
- ↑ R. J. York, R. S. Thiel and E. G. Beaudry, Full-scale experience of direct osmosis concentration applied to leachate management, Sardinia ’99 Seventh International Waste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Sardinia, Italy, 1999.
- ↑ E. G. Beaudry and K. A. Lampi (1990). "Membrane technology for direct osmosis concentration of fruit juices". Food Technology 44: 121.
- ↑ McCutcheon, Jeffrey R.; McGinnis, Robert L.; Elimelech, Menachem (2005). "A novel ammonia—carbon dioxide forward (direct) osmosis desalination process". Desalination 174: 1–11. doi:10.1016/j.desal.2004.11.002.
- ↑ Water Desalination Report, “FO process concentrates oilfield brine”. Published October 8, 2012
Further reading
- Cath, T; Childress, A; Elimelech, M (2006). "Forward osmosis: Principles, applications, and recent developments". Journal of Membrane Science 281: 70–87. doi:10.1016/j.memsci.2006.05.048.
- Duranceau, Steven (July 2012). "Emergence of Forward Osmosis and Pressure-Retarded Osmotic Processes for Drinking Water Treatment". Florida Water Resources Journal: 32–36. Retrieved 14 June 2013.