Fluorosulfuric acid
| Fluorosulfuric acid | |
|---|---|
 |
 |
| IUPAC name Sulfurofluoridic acid | |
| Systematic name Fluorosulfuric acid[citation needed] | |
| Other names Fluorosulfonic acid, | |
| Identifiers | |
| CAS number | 7789-21-1 |
| PubChem | 24603 |
| ChemSpider | 23005 |
| EC number | 232-149-4 |
| UN number | 1777 |
| MeSH | Fluorosulfonic+acid |
| RTECS number | LP0715000 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | HFO3S |
| Molar mass | 100.07 g mol−1 |
| Appearance | Colorless liquid |
| Density | 1.84 g cm-3 |
| Melting point | −87.5 °C; −125.4 °F; 185.7 K |
| Boiling point | 165.4 °C; 329.6 °F; 438.5 K |
| Acidity (pKa) | -10 |
| Basicity (pKb) | 24 |
| Structure | |
| Coordination geometry |
Tetragonal at S |
| Molecular shape | Tetrahedral at S |
| Hazards | |
| MSDS | ICSC 0996 |
| EU Index | 016-018-00-7 |
| EU classification | |
| R-phrases | R20, R35 |
| S-phrases | (S1/2), S26, S45 |
| Related compounds | |
| Related compounds | Antimony pentafluoride Trifluoromethanesulfonic acid Hydrofluoric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
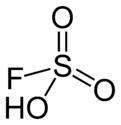
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the formula HSO3F. It is a superacid and one of the strongest acids commercially available. The formula HSO3F emphasizes its relationship to sulfuric acid, H2SO4; HSO3F is a tetrahedral molecule.
Chemical properties
Fluorosulfuric acid is a free-flowing colorless liquid. It is soluble in polar organic solvents (e.g. nitrobenzene, acetic acid, and ethyl acetate), but poorly soluble in nonpolar solvents such as alkanes. Reflecting its strong acidity, it dissolves almost all organic compounds that are even weak proton acceptors.[1] HSO3F hydrolyzes slowly to HF and sulfuric acid. The related triflic acid (CF3SO3H) retains the high acidity of HSO3F but is more hydrolytically stable. The self-ionization of fluorosulfonic acid also occurs:
- 2HSO3F
 [H2SO3F]+ + [SO3F]- K = 4.0 x 10-8 (at 298K)
[H2SO3F]+ + [SO3F]- K = 4.0 x 10-8 (at 298K)
Production
Fluorosulfuric acid is prepared by the reaction of HF and sulfur trioxide:
- SO3 + HF → HSO3F
Alternatively, KHF2 or CaF2 can be treated with oleum at 250 °C. Once freed from HF by sweeping with an inert gas, HSO3F can be distilled in a glass apparatus.[2]
Super-acids
HSO3F is one of the strongest known simple Brønsted acids, although recent work on carborane-based acids have led to still stronger acids.[3] It has an H0 value of −15.1 compared to −12 for sulfuric acid. The combination of HSO3F and the Lewis acid antimony pentafluoride produces "Magic acid," which is a far stronger protonating agent. These acids all fall into the category of "superacids", acids stronger than 100% sulfuric acid.
Applications
HSO3F is useful for regenerating mixtures of HF and H2SO4 for etching lead glass.
HSO3F isomerizes alkanes and the alkylation of hydrocarbons with alkenes,[4] although it is unclear if such applications are of commercial importance. It can also be used as a laboratory fluorinating agent.[2]
Safety
Fluorosulfuric acid is considered to be highly toxic and corrosive. It hydrolyzes to release HF. Addition of water to HSO3F can be violent, similar to the addition of water to sulfuric acid but it is a much more violent process than the addition of water to sulfuric acid.
See also
- Chlorosulfuric acid
- Fluoroboric acid
- Fluoroantimonic acid
- Sulfuryl fluoride
- Methyl fluorosulfonate, an organic ester of FSO3H
- Fluorosulfonylamine FSO2NH2, the formal acid amide
References
- ↑ Olah, G. A.; Prakash, G. K.; Wang, Q.; Li, X.-Y. (2001). "Fluorosulfuric Acid". Encyclopedia of Reagents for Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rf014.
- ↑ 2.0 2.1 Cotton, F. A.; Wilkinson, G. (1980). Advanced Inorganic Chemistry (4th ed.). New York: Wiley. p. 246. ISBN 0-471-02775-8.
- ↑ Juhasz, M.; Hoffmann, S.; Stoyanov, E.; Kim, K. C.; Reed, C. A. (2004). "The Strongest Isolable Acid". Angewandte Chemie International Edition 43 (40): 5352–5355. doi:10.1002/anie.200460005. PMID 15468064.
- ↑ Olah, G.; Farooq, O.; Husain, A.; Ding, N.; Trivedi, N.; Olah, J. (1991). "Superacid HSO3F/HF-Catalyzed Butane Isomerisation". Catalysis Letters 10 (3–4): 239–247. doi:10.1007/BF00772077.