Flow battery
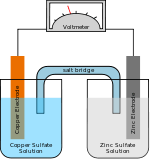
A flow battery is a type of rechargeable battery where rechargeability is provided by two chemical components dissolved in liquids contained within the system and separated by a membrane. Ion exchange (providing flow of electrical current) occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.2 Volts.
A flow battery is technically akin both to a fuel cell and an electrochemical accumulator cell (electrochemical reversibility). While it has technical advantages such as potentially separable liquid tanks and near unlimited longevity over most conventional rechargeables, current implementations are comparatively less powerful and require more sophisticated electronics.
Construction principle
A flow battery is a rechargeable fuel cell in which an electrolyte containing one or more dissolved electroactive elements flow through an electrochemical cell that reversibly converts chemical energy directly to electricity (electroactive elements are "elements in solution that can take part in an electrode reaction or that can be adsorbed on the electrode" ). Additional electrolyte is stored externally, generally in tanks, and is usually pumped through the cell (or cells) of the reactor, although gravity feed systems are also known. Flow batteries can be rapidly "recharged" by replacing the electrolyte liquid (in a similar way to refilling fuel tanks for internal combustion engines) while simultaneously recovering the spent material for re-energization.
In other words, a flow battery is just like an electrochemical cell, with the exception that the ionic solution (electrolyte) is not stored in the cell around the electrodes. Rather, the ionic solution is stored outside of the cell, and can be fed into the cell in order to generate electricity. The total amount of electricity that can be generated depends on the size of the storage tanks. One benefit to this design is that the cell can be recharged simply by changing out the tanks.
Classes of flow batteries
Different classes of flow cells (batteries) have been developed, including redox, hybrid and membraneless. The fundamental difference between conventional batteries and flow cells is that energy is stored as the electrode material in conventional batteries but as the electrolyte in flow cells.
Redox
The redox (reduction-oxidation) cell is a reversible fuel cell in which all electrochemical components are dissolved in the electrolyte. The energy capacity of the redox flow battery is fully independent of its power, because the energy available is related to the electrolyte volume (amount of liquid electrolyte) and the power to the surface area of the electrodes. Redox flow batteries are rechargeable (secondary cells). Because they employ heterogeneous electron transfer rather than solid-state diffusion or intercalation they are more appropriately called fuel cells than batteries. In industrial practice, fuel cells are usually, and unnecessarily, considered to be primary cells, such as the H
2/O
2 system. The unitized regenerative fuel cell on NASA's Helios Prototype is another reversible fuel cell. The European Patent Organisation classifies redox flow cells (H01M8/18C4) as a sub-class of regenerative fuel cells (H01M8/18). Examples of redox flow batteries are the vanadium redox flow battery, polysulfide bromide battery (Regenesys), and uranium redox flow battery. Redox fuel cells are less common commercially although many systems have been proposed.
Hybrid
The hybrid flow battery uses one or more electroactive components deposited as a solid layer. In this case, the electrochemical cell contains one battery electrode and one fuel cell electrode. This type is limited in energy by the surface area of the electrode.
Hybrid flow batteries include the zinc-bromine, zinc-cerium and lead-acid flow batteries.
Membraneless
This battery employs a phenomenon called laminar flow in which two liquids are pumped through a channel. They undergo electrochemical reactions to store or release energy. The solutions stream through in parallel, with little mixing. The flow naturally separates the liquids, eliminating the need for a membrane.
Membranes are often the most costly component and the most unreliable components of batteries, as they can corrode with repeated exposure to certain reactants. The absence of a membrane enabled the use of a liquid bromine solution and hydrogen. This combination is problematic when membranes are used, because they form hydrobromic acid that can destroy the membrane. Both materials are available at low cost.
The design uses a small channel between two electrodes. Liquid bromine flows through the channel over a graphite cathode and hydrobromic acid flows under a porous anode. At the same time, hydrogen gas flows across the anode. The chemical reaction can be reversed to recharge the battery—a first for any membraneless design.
One such membraneless flow battery published in August 2013 produced a maximum power density of 0.795 w/cm2, three times as much power as other membraneless systems— and an order of magnitude higher than lithium-ion batteries.
Organic
In 2013 researchers announced the use of 9,10-anthraquinone-2,7-disulphonic acid (AQDS), a quinone, as a charge carrier in metal-free flow batteries. Each of the carbon-based molecules holds two units of electrical charge, compared with one unit in conventional batteries, implying that a battery could store twice as much energy in a given volume. AQDS undergoes rapid, reversible two-electron/two-proton reduction on a glassy carbon electrode in sulphuric acid. An aqueous flow battery with inexpensive carbon electrodes, combining the quinone/hydroquinone couple with the Br
2/Br−
redox couple, yields a peak galvanic power density exceeding 0.6 W cm−2 at 1.3 A cm−2. Cycling showed >99 per cent storage capacity retention per cycle. The organic anthraquinone species can be synthesized from inexpensive commodity chemicals. This organic approach permits tuning of the reduction potential and solubility by adding functional groups. Adding two hydroxy groups to AQDS increases the open circuit potential of the cell by 11%.
Chemistries
Source^
| Couple | Max. cell voltage (V) | Average electrode power density (W/m2) | Average fluid energy density (W·h/kg) |
|---|---|---|---|
| Bromine-hydrogen | 7,950 | ||
| Iron-tin | 0.62 | <200 | |
| Iron-titanium | 0.43 | <200 | |
| Iron-chrome | 1.07 | <200 | |
| Vanadium-vanadium (sulphate) | 1.4 | ~800 | 25 |
| Vanadium-vanadium (bromide) | 50 | ||
| Sodium/bromine polysulfide | 1.54 | ~800 | |
| Zinc-bromine | 1.85 | ~1,000 | 75 |
| Lead-acid (methanesulfonate) | 1.82 | ~1,000 | |
| Zinc-cerium (methanesulfonate) | 2.43 | <1,200–2,500 |
Advantages and disadvantages
Redox flow batteries, and to a lesser extent hybrid flow batteries, have the advantages of flexible layout (due to separation of the power and energy components), long cycle life (because there are no solid-solid phase transitions), quick response times, no need for "equalisation" charging (the over charging of a battery to ensure all cells have an equal charge) and no harmful emissions. Some types also offer easy state-of-charge determination (through voltage dependence on charge), low maintenance and tolerance to overcharge/overdischarge.
On the negative side, flow batteries are rather complicated in comparison with standard batteries as they may require pumps, sensors, control units and secondary containment vessels. The energy densities vary considerably but are, in general, rather low compared to portable batteries, such as the Li-ion.
Applications
Flow batteries are normally considered for relatively large (1 kW·h – 10 MW·h) stationary applications. These are for
- Load balancing - where the battery is connected to an electrical grid to store excess electrical power during off-peak hours and release electrical power during peak demand periods.
- Storing energy from renewable sources such as wind or solar for discharge during periods of peak demand.
- Peak shaving, where spikes of demand are met by the battery.
- UPS, where the battery is used if the main power fails to provide an uninterrupted supply.
- Power conversion - because all cells share the same electrolyte/s. Therefore, the electrolyte/s may be charged using a given number of cells and discharged with a different number. Because the voltage of the battery is proportional to the number of cells used the battery can therefore act as a very powerful DC/DC converter. In addition, if the number of cells is continuously changed (on the input and/or output side) power conversion can also be AC/DC, AC/AC, or DC/AC with the frequency limited by that of the switching gear.
- Electric vehicles - Because flow batteries can be rapidly "recharged" by replacing the electrolyte, they can be used for applications where the vehicle needs to take on energy as fast as a combustion engined vehicle.
- Stand-alone power system - An example of this is the telecom industry for use in cellphone base stations where there is no grid power available. The battery can be used alongside solar or wind power sources to compensate for their fluctuating power levels and alongside a generator to make the most efficient use of it to save fuel.
See also
- Glossary of fuel cell terms
- Hydrogen technologies
- Load balancing
- Polysulfide bromide battery
- Redox electrode
- Vanadium redox flow battery
- Zinc-cerium hybrid flow battery
- Zinc-bromine hybrid flow battery
- Hydrogen bromine battery
References
- ^ Science-Dictionary.org. "Electroactive Substance" 14 May 2013.
- ^ T. Fujii, T. Hirose, and N. Kondou, in JP patent 55096569 (1979), to Meidensha Electric Mfg. Co. Ltd.
- ^ Bartolozzi, M. (1989). "Development of redox flow batteries. A historical bibliography". Journal of Power Sources 27 (3): 219–234. doi:10.1016/0378-7753(89)80037-0.
- ^ Linden, D.; Reddy, T.B. (2002). Handbook of Batteries (Eds.). McGraw-Hill.
- ^ L. H. Cutler, in US Patent 3607420 (1969), to E.I. du Pont de Nemours and Co.
- ^ Shiokawa, Y.; Yamana, H.; Moriyama, H. (2000). "An Application of Actinide Elements for a Redox Flow Battery". Journal of Nuclear Science and Technology 37 (3): 253. doi:10.1080/18811248.2000.9714891.
- ^ Leung, P. K.; Ponce-De-León, C.; Low, C. T. J.; Shah, A. A.; Walsh, F. C. (2011). "Characterization of a zinc–cerium flow battery". Journal of Power Sources 196 (11): 5174. doi:10.1016/j.jpowsour.2011.01.095.
- ^ W. Borchers, in US patent 567959 (1894)
- ^ W. Nernst, in DE patent 264026 (1912)
- ^ R. M. Keefer, in US patent 3682704 (1970), to Electrocell Ltd.
- ^ Kummer, J. T.; Oei, D. -G. (1985). "A chemically regenerative redox fuel cell. II". Journal of Applied Electrochemistry 15 (4): 619. doi:10.1007/BF01059304.
- ^
- ^ P. M. Spaziante, K. Kampanatsanyakorn, and A. Zocchi, in WO patent 03043170 (2001), to Squirrel Holdings Ltd.
- ^ Talk by John Davis of Deeya energy about their flow battery's use in the telecomms industry on YouTube
- ^ "New rechargeable flow battery enables cheaper, large-scale energy storage". KurzweilAI. doi:10.1038/ncomms3346. Retrieved 2013-08-20.
- ^ Braff, W. A.; Bazant, M. Z.; Buie, C. R. (2013). "Membrane-less hydrogen bromine flow battery". Nature Communications 4. doi:10.1038/ncomms3346.
- ^ Performance Testing of Zinc-Bromine Flow Batteries for Remote Telecom Sites
- ^ Huskinson, B.; Marshak, M. P.; Suh, C.; Er, S. L.; Gerhardt, M. R.; Galvin, C. J.; Chen, X.; Aspuru-Guzik, A. N.; Gordon, R. G.; Aziz, M. J. (2014). "A metal-free organic–inorganic aqueous flow battery". Nature 505 (7482): 195–198. doi:10.1038/nature12909. PMID 24402280.
- ^ "From Harvard, a Cheaper Storage Battery". New York Times. January 8, 2014. Retrieved January 10, 2014.
- ^ "Electric Vehicle Refuelling System (EVRS) used in conjunction with Vanadium Redox Flow Technology". REDT Energy Storage.
- ^ REDT Energy. "Storing Renewable Energy".
External links
- Electropaedia on Flow Batteries
- Research on the uranium redox flow battery
- Improved redox flow batteries for electric cars
| |||||||||||||||||