Flammability limit
Mixtures of dispersed combustible materials (such as gaseous or vaporised fuels, and some dusts) and air will burn only if the fuel concentration lies within well-defined lower and upper bounds determined experimentally, referred to as flammability limits or explosive limits. Combustion can range in violence from deflagration, through detonation, to explosion.
Limits vary with temperature and pressure, but are normally expressed in terms of volume percentage at 25 °C and atmospheric pressure. These limits are relevant both to producing and optimising explosion or combustion, as in an engine, or to preventing it, as in uncontrolled explosions of build-ups of combustible gas or dust. Attaining the best combustible or explosive mixture of a fuel and air (the stoichiometric proportion) is important in internal combustion engines such as gasoline or diesel engines.
The standard reference work is that by Zabetakis using an apparatus developed by the US Bureau of Mines.
Violence of combustion
Combustion can be less or more violent. A deflagration is a propagation of a combustion zone at a velocity less than the speed of sound in the unreacted medium. A detonation is a propagation of a combustion zone at a velocity greater than the speed of sound in the unreacted medium. An explosion is the bursting or rupture of an enclosure or container due to the development of internal pressure from a deflagration or detonation as defined in NFPA 69.
Limits
Lower explosive limit
Lower explosive limit (LEL): The lowest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). The term is considered by many safety professionals to be the same as the lower flammable limit (LFL). At a concentration in air lower than the LEL, gas mixtures are "too lean" to burn. Methane gas has a LEL of 5%. If the atmosphere has less than 5% methane, an explosion cannot occur even if a source of ignition is present.
Percentage reading on combustible air monitors should not be confused with the LEL concentrations. Explosimeters designed and calibrated to a specific gas may show the relative concentration of the atmosphere to the LEL—the LEL being 100%. A 5% displayed LEL reading for methane, for example, would be equivalent to 5.1% multiplied by 5%, or approximately 0.25% methane by volume at 20 degrees C. Control of the explosion hazard is usually achieved by sufficient natural or mechanical ventilation, to limit the concentration of flammable gases or vapors to a maximum level of 25% of their lower explosive or flammable limit. The maximum of a lower explosive limit is also known as the "top LEL."
Upper explosive limit
Upper explosive limit (UEL): Highest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). Concentrations higher than UFL or UEL are "too rich" to burn.
Influence of temperature, pressure and composition
Flammability limits of mixtures of several combustible gases can be calculated using Le Chatelier's mixing rule for combustible volume fractions xi:
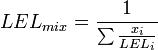
and similar for UEL.
Temperature, pressure, and the concentration of the oxidizer also influences flammability limits. Higher temperature results in lower LFL and higher UFL, while greater pressure increases both values. The effect of pressure is very small at pressures below 10 millibar and difficult to predict, since it has only been studied in internal combustion engines with a turbocharger.
Usually atmospheric air supplies the oxygen for combustion, and limits assume the normal concentration of oxygen in air. Oxygen-enriched atmospheres enhance combustion, lowering the LFL and increasing the UFL, and vice-versa; an atmosphere devoid of an oxidizer is neither flammable nor explosive for any fuel concentration. Significantly increasing the fraction of inert gases in an air mixture, at the expense of oxygen, raises the LFL and decreases the UFL.
Controlling explosive atmospheres
Gas and vapor
Controlling gas and vapor concentrations outside the explosive limits is a major consideration in occupational safety and health. Methods used to control the concentration of a potentially explosive gas or vapor include use of sweep gas, an unreactive gas such as nitrogen or argon to dilute the explosive gas before coming in contact with air. Use of scrubbers or adsorption resins to remove explosive gases before release are also common. Gases can also be maintained safely at concentrations above the UEL, although a breach in the storage container can lead to explosive conditions or intense fires.
Dusts
Dusts also have upper and lower explosion limits, though the upper limits are hard to measure and of little practical importance. Lower explosive limits for many organic materials are in the range of 10–50 g/m³, which is much higher than the limits set for health reasons, as is the case for the LEL of many gases and vapours. Dust clouds of this concentration are hard to see through for more than a short distance, and normally only exist inside process equipment.
Explosion limits also depend on the particle size of the dust involved, and are not intrinsic properties of the material. In addition, a concentration above the LEL can be created suddenly from settled dust accumulations, so management by routine monitoring, as is done with gases and vapours, is of no value. The preferred method of managing combustible dust is by preventing accumulations of settled dust through process enclosure, ventilation, and surface cleaning. However, lower explosion limits may be relevant to plant design.
Examples
The flammable/explosive limits of some gases and vapors are given below. Concentrations are given in percent by volume of air.
- Class IA liquids (flash point less than 73 °F (22.8 °C); boiling point less than 100 °F (37.8 °C) are NFPA 704 flammability rating 4
- Classes IB (flash point less than 73 °F (22.8 °C); boiling point equal to or greater than 100 °F (37.8 °C)) and IC liquids (flash point equal to or greater than 73 °F (22.8 °C), but less than 100 °F (37.8 °C)) are NFPA 704 flammability rating 3
- Classes II (flash point equal to or greater than 100 °F (37.8 °C), but less than 140 °F (60 °C) and IIIA liquids (flash point equal to or greater than 140 °F (60 °C), but less than 200 °F (93.3 °C)) are NFPA 704 flammability rating 2
- Class IIIB liquids (flash point equal to or greater than 200 °F (93.3 °C) are NFPA 704 flammability rating 1
| Substance | LFL/LEL in %
by volume of air |
UFL/UEL in %
by volume of air |
NFPA Class | Flash point | Minimum ignition energy in mJ expressed as percent by volume in air (Note, for many chemicals it |
Autoignition temperature |
|---|---|---|---|---|---|---|
| Acetaldehyde | 4.0 | 57.0 | IA | -39 °C | 0.37 | 175 °C |
| Acetic acid (glacial) | 4 | 19.9 | II | 39 °C to 43 °C | 463 °C | |
| Acetic anhydride | II | 54 °C | ||||
| Acetone | 2.6–3 | 12.8–13 | IB | -17 °C | 1.15 @ 4.5% | 465 °C, 485 °C[2] |
| Acetonitrile | IB | 2 °C | 524 °C | |||
| Acetyl chloride | 7.3 | 19 | IB | 5 °C | 390 °C | |
| Acetylene | 2.5 | 100[3] | IA | Flammable gas | 0.017 @ 8.5% (in pure oxygen 0.0002 @ 40%) | 305 °C |
| Acrolein | 2.8 | 31 | IB | -26 °C | 0.13 | |
| Acrylonitrile | 3.0 | 17.0 | IB | 0 °C | 0.16 @ 9.0% | |
| Allyl chloride | 2.9 | 11.1 | IB | -32 °C | 0.77 | |
| Ammonia | 15 | 28 | IIIB | 11 °C | 680 | 651 °C |
| Arsine | 4.5–5.1[4] | 78 | IA | Flammable gas | ||
| Benzene | 1.2 | 7.8 | IB | -11 °C | 0.2 @ 4.7% | 560 °C |
| 1,3-Butadiene | 2.0 | 12 | IA | -85 °C | 0.13 @ 5.2% | |
| Butane, n-butane | 1.6 | 8.4 | IA | -60 °C | 0.25 @ 4.7% | 420–500 °C |
| n-Butyl acetate, butyl acetate | 1–1.7[2] | 8–15 | IB | 24 °C | 370 °C | |
| Butyl alcohol, butanol | 1 | 11 | IC | 29 °C | ||
| n-Butanol | 1.4[2] | 11.2 | IC | 35 °C | 340 °C | |
| n-Butyl chloride, 1-chlorobutane | 1.8 | 10.1 | IB | -6 °C | 1.24 | |
| n-Butyl mercaptan | 1.4[5] | 10.2 | IB | 2 °C | 225 °C | |
| Butyl methyl ketone, 2-hexanone | 1[6] | 8 | IC | 25 °C | 423 °C | |
| Butylene, 1-butylene, 1-butene | 1.98[4] | 9.65 | IA | -80 °C | ||
| Carbon disulfide | 1.0 | 50.0 | IB | -30 °C | 0.009 @ 7.8% | 90 °C |
| Carbon monoxide | 12[4] | 75 | IA | -191 °C Flammable gas | 609 °C | |
| Chlorine monoxide | IA | Flammable gas | ||||
| 1-Chloro-1,1-difluoroethane | 6.2 | 17.9 | IA | -65 °C Flammable gas | ||
| Cyanogen | 6.0–6.6[7] | 32–42.6 | IA | Flammable gas | ||
| Cyclobutane | 1.8 | 11.1 | IA | -63.9 °C[8] | 426.7 °C | |
| Cyclohexane | 1.3 | 7.8–8 | IB | -18 °C to -20 °C[9] | 0.22 @ 3.8% | 245 °C |
| Cyclohexanol | 1 | 9 | IIIA | 68 °C | 300 °C | |
| Cyclohexanone | 1–1.1 | 9–9.4 | II | 43.9–44 °C | 420 °C[10] | |
| Cyclopentadiene[11] | IB | 0 °C | 0.67 | 640 °C | ||
| Cyclopentane | 1.5–2 | 9.4 | IB | -37 to -38.9 °C[12][13] | 0.54 | 361 °C |
| Cyclopropane | 2.4 | 10.4 | IA | -94.4 °C[14] | 0.17 @ 6.3% | 498 °C |
| Decane | 0.8 | 5.4 | II | 46.1 °C | 210 °C | |
| Diborane | 0.8 | 88 | IA | -90 °C Flammable gas[15] | 38 °C | |
| o-Dichlorobenzene, 1,2-dichlorobenzene | 2[16] | 9 | IIIA | 65 °C | 648 °C | |
| 1,1-Dichloroethane | 6 | 11 | IB | 14 °C | ||
| 1,2-Dichloroethane | 6 | 16 | IB | 13 °C | 413 °C | |
| 1,1-Dichloroethene | 6.5 | 15.5 | IA | -10 °C Flammable gas | ||
| Dichlorofluoromethane | 54.7 | Non flammable,[17] -36.1 °C[18] | 552 °C | |||
| Dichloromethane, methylene chloride | 16 | 66 | Non flammable | |||
| Dichlorosilane | 4 - 4.7 | 96 | IA | -28 °C | 0.015 | |
| Diesel fuel | 0.6 | 7.5 | IIIA | >62 °C (143 °F) | 210 °C | |
| Diethanolamine | 2 | 13 | IB | 169 °C | ||
| Diethylamine | 1.8 | 10.1 | IB | -23 to -26 °C | 312 °C | |
| Diethyl disulfide | 1.2 | II | 38.9 °C[19] | |||
| Diethyl ether | 1.9–2 | 36–48 | IA | -45 °C | 0.19 @ 5.1% | 160–170 °C |
| Diethyl sulfide | IB | -10 °C[20] | ||||
| 1,1-Difluoroethane | 3.7 | 18 | IA | -81.1 °C[21] | ||
| 1,1-Difluoroethylene | 5.5 | 21.3 | -126.1 °C[22] | |||
| Diisobutyl ketone | 1 | 6 | 49 °C | |||
| Diisopropyl ether | 1 | 21 | IB | -28 °C | ||
| Dimethylamine | 2.8 | 14.4 | IA | Flammable gas | ||
| 1,1-Dimethylhydrazine | IB | |||||
| Dimethyl sulfide | IA | -49 °C | ||||
| Dimethyl sulfoxide | 2.6–3 | 42 | IIIB | 88–95 °C | 215 °C | |
| 1,4-Dioxane | 2 | 22 | IB | 12 °C | ||
| Epichlorohydrin | 4 | 21 | 31 °C | |||
| Ethane | 3[4] | 12–12.4 | IA | Flammable gas -135 °C | 515 °C | |
| Ethanol, ethyl alcohol | 3–3.3 | 19 | IB | 12.8 °C (55 °F) | 365 °C | |
| 2-Ethoxyethanol | 3 | 18 | 43 °C | |||
| 2-Ethoxyethyl acetate | 2 | 8 | 56 °C | |||
| Ethyl acetate | 2 | 12 | IA | -4 °C | 460 °C | |
| Ethylamine | 3.5 | 14 | IA | -17 °C | ||
| Ethylbenzene | 1.0 | 7.1 | 15–20 °C | |||
| Ethylene | 2.7 | 36 | IA | 0.07 | 490 °C | |
| Ethylene glycol | 3 | 22 | 111 °C | |||
| Ethylene oxide | 3 | 100 | IA | −20 °C | ||
| Ethyl chloride | 3.8[4] | 15.4 | IA | −50 °C | ||
| Ethyl mercaptan | IA | |||||
| Fuel oil No.1 | 0.7[4] | 5 | ||||
| Furan | 2 | 14 | IA | -36 °C | ||
| Gasoline (100 octane) | 1.4 | 7.6 | IB | < −40 °C (−40 °F) | 246–280 °C | |
| Glycerol | 3 | 19 | 199 °C | |||
| Heptane, n-heptane | 1.05 | 6.7 | -4 °C | 0.24 @ 3.4% | 204–215 °C | |
| Hexane, n-hexane | 1.1 | 7.5 | -22 °C | 0.24 @ 3.8% | 225 °C, 233 °C[2] | |
| Hydrogen | 4/18.3[23] | 75/59 | IA | Flammable gas | 0.016 @ 28% (in pure oxygen 0.0012) | 500–571 °C |
| Hydrogen sulfide | 4.3 | 46 | IA | Flammable gas | 0.068 | |
| Isobutane | 1.8[4] | 9.6 | IA | Flammable gas | 462 °C | |
| Isobutyl alcohol | 2 | 11 | 28 °C | |||
| Isophorone | 1 | 4 | 84 °C | |||
| Isopropyl alcohol, isopropanol | 2[4] | 12 | IB | 12 °C | 398–399 °C; 425 °C[2] | |
| Isopropyl chloride | IA | |||||
| Kerosene Jet A-1 | 0.6–0.7 | 4.9–5 | II | >38 °C (100 °F) as jet fuel | 210 °C | |
| Lithium hydride | IA | |||||
| 2-Mercaptoethanol | IIIA | |||||
| Methane (natural gas) | 4.4–5 | 15–17 | IA | Flammable gas | 0.21 @ 8.5% | 580 °C |
| Methyl acetate | 3 | 16 | -10 °C | |||
| Methyl alcohol, methanol | 6–6.7[4] | 36 | IB | 11 °C | 385 °C; 455 °C[2] | |
| Methylamine | IA | 8 °C | ||||
| Methyl chloride | 10.7[4] | 17.4 | IA | -46 °C | ||
| Methyl ether | IA | −41 °C | ||||
| Methyl ethyl ether | IA | |||||
| Methyl ethyl ketone | 1.8[4] | 10 | IB | -6 °C | 505–515 °C[2] | |
| Methyl formate | IA | |||||
| Methyl mercaptan | 3.9 | 21.8 | IA | -53 °C | ||
| Mineral spirits | 0.7[2] | 6.5 | 38–43 °C | 258 °C | ||
| Morpholine | 1.8 | 10.8 | IC | 31–37.7 °C | 310 °C | |
| Naphthalene | 0.9[4] | 5.9 | IIIA | 79–87 °C | ||
| Neohexane | 1.19[4] | 7.58 | −29 °C | 425 °C | ||
| Nickel tetracarbonyl | 2 | 34 | 4 °C | 60 °C | ||
| Nitrobenzene | 2 | 9 | IIIA | 88 °C | ||
| Nitromethane | 7.3 | 22.2 | 35 °C | 379 °C | ||
| Octane | 1 | 7 | 13 °C | |||
| iso-Octane | 0.79 | 5.94 | ||||
| Pentane | 1.5 | 7.8 | IA | -40 to -49 °C | as 2-Pentane 0.18 @ 4.4% | 260 °C |
| n-Pentane | 1.4 | 7.8 | IA | 0.28 @ 3.3% | ||
| iso-Pentane | 1.32[4] | 9.16 | IA | 420 °C | ||
| Phosphine | IA | |||||
| Propane | 2.1 | 9.5–10.1 | IA | Flammable gas | 0.25 @ 5.2% (in pure oxygen 0.0021) | 480 °C |
| Propyl acetate | 2 | 8 | 13 °C | |||
| Propylene | 2.0 | 11.1 | IA | -108 °C | 0.28 | 458 °C |
| Propylene oxide | 2.3 | 36 | IA | |||
| Pyridine | 2 | 12 | 20 °C | |||
| Silane | 1.5[4] | 98 | IA | <21 °C | ||
| Styrene | 1.1 | 6.1 | IB | 31–32.2 °C | 490 °C | |
| Tetrafluoroethylene | IA | |||||
| Tetrahydrofuran | 2 | 12 | IB | -14 °C | 321 °C | |
| Toluene | 1.2–1.27 | 6.75–7.1 | IB | 4.4 °C | 0.24 @ 4.1% | 480 °C; 535 °C[2] |
| Triethylborane | -20 °C | -20 °C | ||||
| Trimethylamine | IA | Flammable gas | ||||
| Trinitrobenzene | IA | |||||
| Turpentine | 0.8[24] | IC | 35 °C | |||
| Vegetable oil | IIIB | 327 °C (620 °F) | ||||
| Vinyl acetate | 2.6 | 13.4 | −8 °C | |||
| Vinyl chloride | 3.6 | 33 | ||||
| Xylenes | 0.9–1.0 | 6.7–7.0 | IC | 27–32 °C | 0.2 | |
| m-Xylene | 1.1[2] | 7 | IC | 25 °C | 525 °C | |
| o-Xylene | IC | 17 °C | ||||
| p-Xylene | 1.0 | 6.0 | IC | 27.2 °C | 530 °C |
See also
References
- ↑ Britton, L. G “Using Material Data in Static Hazard Assessment.” as found in NFPA 77 - 2007 Appendix B
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ↑ Matheson Gas Products. Matheson Gas Data Book. p. 443. Retrieved 2013-10-30.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 4.10 4.11 4.12 4.13 4.14 "Gases - Explosive and Flammability Concentration Limits". Retrieved 2013-09-09.
- ↑ n-BUTYL MERCAPTAN ICSC: 0018
- ↑ 2-HEXANONE ICSC:0489
- ↑ Cyanogen
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 211
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 216
- ↑ CYCLOHEXANONE ICSC: 0425
- ↑ MSDS Cyclopentadiene
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 221
- ↑ CYCLOPENTANE ICSC: 0353
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 226
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 244
- ↑ Walsh (1989) Chemical Safety Data Sheets, Roy. Soc. Chem., Cambridge.
- ↑ Encyclopedia.airliquide.com
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 266
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 281
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 286
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 296
- ↑ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 301
- ↑ http://environmentalchemistry.com/yogi/periodic/H.html
- ↑ Combustibles
Further reading
- David R. Lide, Editor-in-Chief; CRC Handbook of Chemistry and Physics, 72nd edition; CRC Press; Boca Raton, Florida; 1991; ISBN 0-8493-0565-9