Fengabine
From Wikipedia, the free encyclopedia
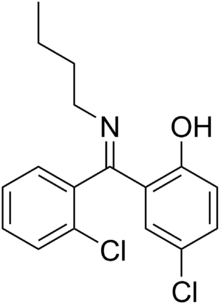 | |
|---|---|
| Systematic (IUPAC) name | |
| (6Z)-6-[butylamino-(2-chlorophenyl)methylene]-4-chloro-cyclohexa-2,4-dien-1-one | |
| Clinical data | |
| Legal status | Uncontrolled |
| Routes | Oral |
| Identifiers | |
| CAS number | 80018-06-0 |
| ATC code | None |
| PubChem | CID 5362066 |
| ChemSpider | 4514924 |
| UNII | YQG0NJI5A7 |
| KEGG | D04149 |
| Chemical data | |
| Formula | C17H17Cl2NO |
| Mol. mass | 322.23 g/mol |
Fengabine (SL-79,229) is a drug which was investigated as an antidepressant but was never marketed.[1][2] Its mechanism of action is unknown, but its antidepressant effects are reversed by GABAA receptor antagonists like bicuculline and it has hence been labeled as GABAergic; however, it does not actually bind to GABA receptors, nor does it inhibit GABA-T.[1][2] In clinical trials, fengabine's efficacy was comparable to that of the tricyclic antidepressants, but with a more rapid onset of action and much less side effects.[3][4][5] Notably, fengabine lacks any sedative effects.[4]
See also
References
- ↑ 1.0 1.1 Lloyd KG, Zivkovic B, Sanger D, Depoortere H, Bartholini G (April 1987). "Fengabine, a novel antidepressant GABAergic agent. I. Activity in models for antidepressant drugs and psychopharmacological profile". The Journal of Pharmacology and Experimental Therapeutics 241 (1): 245–50. PMID 3033203.
- ↑ 2.0 2.1 Scatton B, Lloyd KG, Zivkovic B, et al. (April 1987). "Fengabine, a novel antidepressant GABAergic agent. II. Effect on cerebral noradrenergic, serotonergic and GABAergic transmission in the rat". The Journal of Pharmacology and Experimental Therapeutics 241 (1): 251–7. PMID 3033204.
- ↑ Magni G, Garreau M, Orofiamma B, Palminteri R (1989). "Fengabine, a new GABAmimetic agent in the treatment of depressive disorders: an overview of six double-blind studies versus tricyclics". Neuropsychobiology 20 (3): 126–31. PMID 2668780.
- ↑ 4.0 4.1 Nielsen NP, Cesana B, Zizolfi S, Ascalone V, Priore P, Morselli PL (November 1990). "Therapeutic effects of fengabine, a new GABAergic agent, in depressed outpatients: a double-blind study versus clomipramine". Acta Psychiatrica Scandinavica 82 (5): 366–71. PMID 2281807.
- ↑ Fairweather DB, Kerr JS, Hilton S, Hindmarch I (March 1993). "A placebo controlled double-blind evaluation of the pharmacodynamics of fengabine vs amitriptyline following single and multiple doses in elderly volunteers". British Journal of Clinical Pharmacology 35 (3): 278–83. PMC 1381575. PMID 8471403.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.