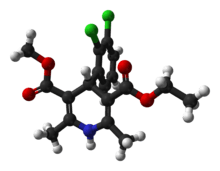
Felodipine
/-/(%C2%B1)-Felodipine_formulae_V.1.svg.png) | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| (RS)-3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | |
| Clinical data | |
| Trade names | Plendil |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a692016 |
| Pregnancy cat. | C (US) |
| Legal status | ℞ Prescription only |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 15% [1] |
| Metabolism | Hepatic |
| Half-life | ?? |
| Excretion | Renal |
| Identifiers | |
| CAS number | 72509-76-3 |
| ATC code | C08CA02 |
| PubChem | CID 3333 |
| IUPHAR ligand | 4190 |
| DrugBank | DB01023 |
| ChemSpider | 3216 |
| UNII | OL961R6O2C |
| KEGG | D00319 |
| ChEBI | CHEBI:585948 |
| ChEMBL | CHEMBL1480 |
| Chemical data | |
| Formula | C18H19Cl2NO4 |
| Mol. mass | 384.259 g/mol |
| SMILES
| |
| |
| | |
Felodipine is a calcium channel blocker (calcium antagonist), a drug used to control hypertension (high blood pressure). It is marketed under the brand name Plendil by AstraZeneca and Renedil by Sanofi-Aventis. The formulation patent for the substance expired in 2007.
AstraZeneca dropped Plendil from its support and AZ&Me free Rx access program in October 2008.
Interactions
Studies dating back to 1989 have suggested that felodipine in combination with grapefruit juice can cause toxic effects. Oral administration of felodipine is first metabolized in the gastrointestinal tract and liver by the enzyme CYP3A4. Grapefruit juice contains bergamottin which is found to have an inhibiting effect over this enzyme and as a result the bioavailability of the drug increases, raising the risk for abnormal side effects.[2]
Contraindications and cautions
Contraindicated with allergy to felodipine or other calcium channel blockers, sick sinus syndrome, heart block (second and third degree), lactation. Use cautiously with pregnancy, impaired hepatic function.
Synthesis
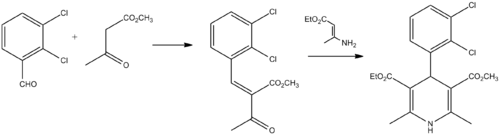
Berntsson, P. B.; Carlsson, A. I.; Gaarder, J. O.; Ljung, B. R.; 1981, U.S. Patent 4,264,611.
References
- ↑ AstraZeneca MI Department, 16th April 2010.
- ↑ Jawad Kiani, Sardar Z Imam (October 30, 2007). "Medicinal importance of grapefruit juice and its interaction with various drugs". Nutr J. 6 (33): 33. doi:10.1186/1475-2891-6-33. PMC 2147024. PMID 17971226. Retrieved 2008-04-09..