Febuxostat
 | |
|---|---|
| Systematic (IUPAC) name | |
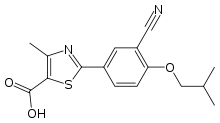
| 2-(3-cyano-4-isobutoxyphenyl)-4-methyl- 1,3-thiazole-5-carboxylic acid | |
| Clinical data | |
| Trade names | Uloric |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a609020 |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy cat. | C |
| Legal status | ℞-only (US) |
| Routes | oral |
| Pharmacokinetic data | |
| Bioavailability | ~49% absorbed |
| Protein binding | ~99% to albumin |
| Metabolism | via CYP1A2, 2C8, 2C9, UGT1A1, 1A3, 1A9, 2B7 |
| Half-life | ~5-8 hours |
| Excretion | urine (~49% mostly as metabolites, 3% as unchanged drug); feces (~45% mostly as metabolites, 12% as unchanged drug) |
| Identifiers | |
| CAS number | 144060-53-7 |
| ATC code | M04AA03 |
| PubChem | CID 134018 |
| ChemSpider | 118173 |
| UNII | 101V0R1N2E |
| KEGG | D01206 |
| ChEMBL | CHEMBL1164729 |
| Chemical data | |
| Formula | C16H16N2O3S |
| Mol. mass | 316.374 g/mol |
| SMILES
| |
| |
| | |
Febuxostat (INN; trade names: Adenuric in Europe and New Zealand, Febutaz in India and Takeda's Uloric in the US) is a urate lowering drug, an inhibitor of xanthine oxidase that is indicated for use in the treatment of hyperuricemia and chronic gout.[1]
Febuxostat received marketing approval by the European Medicines Agency on April 21, 2008[2] and was approved by the U.S. Food and Drug Administration on February 16, 2009.[3]
A study comparing febuxostat to allopurinol found that more individuals treated with febuxostat had decreased levels of uric acid, but there was no difference in the amount of initial gout flares or the surface area of gout tophi.[4]
A committee of the British National Institute for Health and Clinical Excellence concluded that although febuxostat had been shown to be more effective than fixed-dose (300 mg) allopurinol in lowering serum uric acid concentration, it had not been shown to be clinically more efficacious or cost effective compared with allopurinol when taken to control uric acid levels (up to 900 mg). However, the committee recommended febuxostat for people who are intolerant of allopurinol.[5]
Mechanism of action
Febuxostat is a non-purine selective inhibitor of xanthine oxidase. It works by non-competitively blocking the molybdenum pterin center which is the active site on xanthine oxidase. Xanthine oxidase is needed to successively oxidize both hypoxanthine and xanthine to uric acid. Hence, febuxostat inhibits xanthine oxidase, therefore reducing production of uric acid. Febuxostat inhibits both oxidized as well as reduced form of xanthine oxidase because of which febuxostat cannot be easily displaced from the molybdenum pterin site.
Clinical efficacy
Many long and short-term clinical trials have proved the efficacy of Febuxostat in the treatment of gout and lowering uric acid levels. In these studies Febuxostat was found to be superior to Allopurinol in reducing the serum uric acid levels. Some notable landmark clinical trials are FACT, APEX, EXCEL, FOCUS and CONFIRMS.
Febuxostat versus Allopurinol Controlled Trial (FACT): 760 patients with gout and a sUA >8.0 mg/dl were randomly assigned to receive either febuxostat 80 or 120mg or allopurinol 300mg once daily for 52 weeks.The primary endpoint was the proportion of patients to achieve a sUA concentration below 6.0 mg/dl at the last three monthly measurements. The primary endpoint was achieved in 53% of patients receiving 80 mg febuxostat, 62% of patients receiving 120mg and 21% of those receiving allopurinol (p <0.001 for each febuxostat group compared with allopurinol). [6]
Allopurinol Placebo controlled Efficacy study of febuXostat (APEX): The APEX trial was a head-to-head phase III controlled clinical trial for gout, with a total of 1072 patients with sUA levels higher than 8.0 mg/dl. Patients were randomized to a once daily fixed dose of placebo; febuxostat 80mg,120mg, or 240 mg; or allopurinol 300mg or 100mg, depending on their baseline serum creatinine (≤1.5 mg/dl or ≥1.6 to <2.0 mg/dl, respectively). The primary endpoint for the trial was the proportion of subjects with sUA levels below 6.0 mg/dl at each of the last three visits. After 1 year of treatment, 82% of the patients in all febuxostat groups achieved sUA levels below 6.0 mg/dl, compared with 39% of the patients in both allopurinol groups. In groups with moderate renal impairment the primary endpoint was achieved by 44% receiving febuxostat 80mg, 45% receiving 120mg, and 60% receiving 240mg, compared with 0% in the allopurinol and placebo groups.[7]
Febuxostat Comparative EXtension Long-Term study (EXCEL): The EXCEL trial was the other long-term trial that assessed the clinical efficacy and safety of febuxostat against allopurinol. In this study, 1086 patients were enrolled to receive fixed daily doses of febuxostat 80mg or 120 mg, or allopurinol 300 mg. Dose adjustments were allowed during the first 6 months to maintain sUA levels between 3.0 and 6.0 mg/dl. The primary endpoint, as in most of the trials, was maintenance of sUA below 6.0 mg/dl and other measures assessed were flares requiring treatment, tophus size and safety profile. After the first month of treatment, nearly 80% of patients receiving either febuxostat dose achieved sUA less than 6 mg/dl, compared with only 46% of subjects on allopurinol. After ULT reassignment, more than 80% of all remaining subjects maintained target levels of sUA at each visit. Maintenance of sUA below 6.0 mg/dl resulted in baseline tophus resolution in 46%, 36%, and 29% of subjects on febuxostat 80mg, 120mg and allopurinol, respectively. In addition, gout flares were significantly reduced, obviating the need for gout flare therapy. Overall adverse events did not show significant differences among groups. [8]
Febuxostat Open Label of Urate-Lowering Efficacy and Safety (FOCUS):The FOCUS trial was a 5-year extension study that assessed reduction and maintenance of sUA levels below 6.0 mg/dl as the primary efficacy endpoint. A total of 116 patients were initially enrolled to receive a dose of 80mg febuxostat with dose adjustment to either 40 or 120mg between weeks 4 and 24. At 5 years, 50% of patients were discontinued prematurely from the study with no apparent relation to adverse events; among the remaining 50% of patients, 93% maintained a sUA level below 6.0 mg/dl at 5 years. There was a clear association with no gout flares in these patients and most patients also had tophus resolution. [9]
Cardiovascular adverse events (AE)
At least one CV AE was investigator-reported in 5% of all subjects: 5%, 5%, and 6% of subjects in the febuxostat 40 mg, febuxostat 80 mg, and allopurinol groups, respectively. No difference between treatment groups in specific CV AEs was detected. Pre-specified adjudication of all deaths and all AEs reported to be CV system-related identified a total of six subjects experiencing an adjudicated APTC (Antiplatelet Trialists Collaboration) event: zero in the febuxostat 40 mg group, three in the febuxostat 80 mg group and three in the allopurinol group. All subjects experiencing an adjudicated APTC event had prior medical histories of or underlying risk factors for CVD. Differences in the rates of adjudicated non-APTC CV events between treatment groups were not significant. [10]
Dosage and administration

For treatment of hyperuricemia in patients with gout, febuxostat is recommended at 40 mg or 80 mg once daily. No dose adjustment is necessary when administering febuxostat in patients with mild to moderate renal and hepatic impairment.[11]
Side effects
The adverse effects associated with febuxostat therapy include nausea, diarrhea, arthralgia, headache, increased hepatic serum enzyme levels and rash.[11][12]
Drug interactions
Febuxostat is contraindicated with concomitant use of theophylline and chemotherapeutic agents, namely azathioprine and 6-mercaptopurine, because it could increase blood plasma concentrations of these drugs, and therefore their toxicity.[13] However, according to 2012 data, theophylline can be administered in usual doses in combination with febuxostat, as the latter does not affect the plasma pharmacokinetics of theophylline. While it does affect the pharmacokinetics of 1-methylxanthine and 1-methyluric acid, these do not have any pharmacological effects.[14]
References
- ↑ Stamp LK, O'Donnell JL, Chapman PT (2007). "Emerging therapies in the long-term management of hyperuricaemia and gout". Internal medicine journal 37 (4): 258–66. doi:10.1111/j.1445-5994.2007.01315.x. PMID 17388867.
- ↑ "Adenuric (febuxostat) receives marketing authorisation in the European Union". Retrieved 2008-05-28.
- ↑ "Uloric Approved for Gout". U.S. News and World Report. Retrieved 2009-02-16.
- ↑ Becker MA, Schumacher HR, Wortmann RL, et al. (December 2005). "Febuxostat compared with allopurinol in patients with hyperuricemia and gout". N. Engl. J. Med. 353 (23): 2450–61. doi:10.1056/NEJMoa050373. PMID 16339094.
- ↑ Febuxostat for the management of hyperuricaemia in people with gout (TA164) Chapter 4. Consideration of the evidence
- ↑ "Ther Adv Musculoskel Dis (2011) 3(5) 245 - 253
- ↑ "Ther Adv Musculoskel Dis (2011) 3(5) 245 - 253
- ↑ "Ther Adv Musculoskel Dis (2011) 3(5) 245 - 253
- ↑ "Ther Adv Musculoskel Dis (2011) 3(5) 245 - 253
- ↑ Becker et al. Arthritis Research & Therapy 2010, 12:R63
- ↑ 11.0 11.1 ULORIC package insert, Takeda Pharmaceuticals America, Deerfield, IL, February, 2009.
- ↑ Love BL, Barrons R, Veverka A, Snider KM (2010). "Urate-lowering therapy for gout: focus on febuxostat". Pharmacotherapy 30 (6): 594–608. doi:10.1592/phco.30.6.594. PMID 20500048.
- ↑ Ashraf Mozayani, Lionel Raymon (2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science+Business Media.
- ↑ Tsai, M; Wu, JT; Gunawardhana, L; Naik, H (2012). "The effects of xanthine oxidase inhibition by febuxostat on the pharmacokinetics of theophylline". International journal of clinical pharmacology and therapeutics 50 (5): 331–7. PMID 22541837.
| ||||||||||||||||||||||||||