Fate mapping
In developmental biology, fate mapping is a method of understanding the embryonic origin of various tissues in the adult organism by establishing the correspondence between individual cells (or groups of cells)) at one stage of development, and their progeny at later stages of development. When carried out at single-cell resolution, this process is termed cell lineage tracing.
History
The first attempts at fate mapping were made by inferences based on the examination of embryos that had been fixed, sectioned, and stained at different developmental timepoints. The disadvantage of this technique was that observation of single points in developmental time provide only snapshots of what cell movements are actually occurring and what fates are being assigned. Early embryologists thus had to infer which cells became what tissues at later stages.
Early embryologists used "vital dyes" (which would stain but not harm the cells) to follow movements of individual cells or groups of cells over time in Xenopus frog embryos. The tissue(s) to which the cells contribute would thus be labeled and visible in the adult organism. The first person to develop and use this technique to study cell fate was embryologist Walter Vogt in 1929. Vogt used small chips of agar impregnated with a vital dye, (such as Nile Blue or Nile Red) which he placed on a particular cell or population of cells in Xenopus embryos until the dye absorbed into the yolk platelets within the desired cell(s). Once the cells were effectively labeled, the agar chip could be removed and the embryo was allowed to develop normally. With this method, Vogt was able to discern movements of particular cell populations and the ultimate organ or tissue into which they integrated. Although innovative for the time, this technique is limiting in that the size of a chip of agar may not accommodate single-cell resolution studies at later stages of development, since successive cell divisions will yield smaller cells (until the embryo develops into a larval form that can eat, and thereby grow larger). Additionally, the cell or cell population of interest must be superficial, since the agar chip with the dye must be placed on the surface of the embryo.
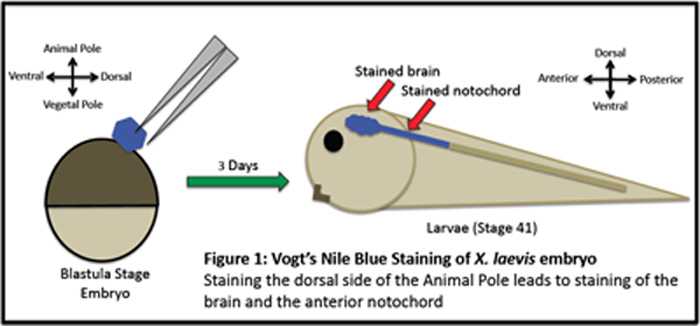
The information Vogt gathered from his tracing experiments of distinct cells and populations of cells in Xenopus was then pooled to construct a fate map. The map was a representation of an early-stage embryo (such as a blastula) that has particular regions highlighted which are known to give rise to specific tissues in the adult organism. For instance, in Figure 1, Nile Blue staining of a 32-cell blastula at the dorsal side of the animal pole yields a blue-stained brain and (depending on the size of the agar chip) may also stain the anterior portion of the notochord.
In 1978, David Weisblat and colleagues in Gunther Stent's lab at Berkeley improved the technique of single-cell resolution fate mapping by injection of horseradish peroxidase (HRP) enzyme, and later fluorescent peptides (1980), into individual cells in Helobdella triserialis (leech) embryos during early development. All progeny of the injected cells could later be discerned by staining for HRP using benzidine substrate or visualized by fluorescence microscopy. This technique allowed the experimenter greater control and selectivity over what cell was labeled and traced. However, the opaque character of the HRP stain prevented use of vital dye nuclear counter-stains such as Hoechst 33258 (blue) to observe the mitotic state of the injected cell's progeny. Also, embryos had to be fixed in order to stain for the HRP, thus allowing only a single timepoint view of each individual leech embryo injected. The use of fluorescent peptides such as Rhodamine-D-protein (red, RDP) and Fluorescein-D-protein (yellow/green, FDP) conjugated to large carrier molecules to prevent diffusion through cell gap junctions, alleviated several of the shortcomings of HRP injection. Leech embryos injected with the fluorescent tracers could be visualized, and images collected of the same specimen at multiple timepoints, without fixation. The fluorescent tracers could also be combined with nuclear Hoechst staining to visualize the mitotic status of the progeny of injected cells. Seth Blair, also in Stent's lab, introduced a novel ablation technique that could be used in tandem with lineage tracing to pursue the questions relating to developmental potential changes in cell fate in experimentally perturbed embryos that were first raised by Roux and Driesch (1980). For this purpose, specific cells were ablated by microinjection with Pronase (an enzyme that digests proteins) to ablate the cell; later modifications of this technique employed DNase or the ricin A chain. The single-cell injection technique is now also in use by researchers studying other model organisms such as Xenopus (frogs), Danio rerio (zebrafish), and Caenorhabditis elegans (worms).
Genetic cell lineage tracing became an extremely powerful approach when Nat Sternberg and Daniel Hamilton identified a topoisomerase in P1 bacteriophage called Cre recombinase, which has site-specific (loxP site) recombination activity (1981). Subsequent studies have used the Cre-lox system in transgenic mice and zebrafish to create tissue specific conditional knockouts. In order to conditionally ablate a tissue or cell population of interest, one can create a transgenic mouse using a construct containing the promoter of a gene that is specific to the target cell population, so that the Cre recombinase is only expressed in that tissue type. A second transgenic line must be created, in which loxP recognition sites flank a portion of a particular critical "housekeeping" gene such as DNA polymerase-β. Each transgene is completely harmless by itself; however, when the two strains are crossed, the Cre expressed in the target cell population will cause the excision of DNA pol-β in those cells, which will die. Use of the Cre system instead of injected Pronase ensures that when the targeted cell(s) dies, its intracellular contents will not harm neighboring cells since the Cre is innocuous by itself and outside the cell, whereas Pronase can damage other cells and the extracellular matrix and thus may have nonspecific effects on development. This approach allows tissue-specific targeted ablation studies, which are important for understanding the importance of particular progenitor cells in the establishment and functionality of adult tissues.
However, in some cases a particular gene of interest is turned on multiple times during development, and Cre-mediated ablation during that first wave of expression is lethal. In this scenario, it is possible to use an inducible version of the transgene: the Cre-ERt loxP approach. Here, the Cre-ERt is a fusion of the recombinase and Tamoxifen-responsive estrogen receptor (ERt). Cre-ERt will be expressed in the cytoplasm of cells expressing its upstream promoter, but will not translocate to the nucleus to conduct recombination until the animal is dosed with Tamoxifen. This tightly-regulated genetic approach to ablation studies has been an invaluable tool for learning about the hierarchy of cell fate during embryogenesis – as well as many other fields of research.
Cre-lox can also be used to permanently, fluorescently label cells by using a transgenic which contains a ubiquitous promoter such as β-actin driving a super-stop sequence flanked by loxP sites, upstream of a fluorescent protein sequence such as RFP. In this fashion, Julien Bertrand from David Traver's lab and Neil Chi discovered the endothelial-origin of hematopoietic (blood) stem cells in zebrafish by using a transgenic with an endothelial promoter, kdrl, driving Cre recombinase crossed to β-actin loxP transgenic fish (2010). Thus, in the presence of Cre, all endothelium-derived cells became indelibly fluorescent red since the super-stop was removed from the genome, and RFP will be expressed under the control of β-actin in all cellular progeny. Indeed, they found that nearly all hematopoietic stem cells and differentiated blood cells were RFP+, indicating their embryonic endothelial origin (See Figure 2). Similar site-directed recombination technology such as FLP-FRT recombination (Flippase and Flippase recognition target sites) is extremely powerful in cell fate mapping, ablation studies, and genetic mosaic analysis; this tool is also used heavily in studies in animal systems as divergent as mice and flies.

Work in other animal models such as the nematode C. elegans was done with single-cell resolution prior to the widespread use of injectable cell tracers. The rapid embryonic development and transparent nature of the nematode allowed the construction of a hierarchical fate map of each mitotic event, from the single-cell zygote to the multicellular adult worm by J. E. Sulston and colleagues in 1982. This cell lineage map was based entirely on observation using Nomarski optics, with no dyes.
New Techniques
More recently, scientists have developed new tools inspired by past approaches. The use of fluorescent peptide tracers can be helpful, but in order to extend fate mapping to later stages when cells are smaller and thus difficult to consistently and selectively inject (unlike the 0.5mm leech embryo), modifications were made. Chemically-"caged" fluorescent peptides, such as caged Rhodamine-dextran or caged Fluorescein-dextran (FITC), have been developed to be non-fluorescent until hit with an ultraviolet (450 nm) laser, which "uncages" the compound and causes it to fluoresce. This tool was particularly well received in the zebrafish community since it is ideal to inject caged compound into a freshly fertilized, single-cell embryo that will rapidly develop over 24 hours into a transparent swimming larvae with approximately 30,000 cells. Injection of the compound into the single-cell embryo allows uniform distribution throughout all the cells of the developing embryo, and the dextran carrier, developed by Jochen Braun and Bob Glimich prevents diffusion between cells through gap junctions, which are common during embryogenesis. At a given stage of development, one can use a UV laser to uncage the compound in a distinct cell or set of cells, effectively labeling them red (Rhodamine) or green (FITC). The embryos can be allowed to develop normally until a later time, at which point they can be imaged for red or green fluorescence in the progeny of the uncaged cells. However, it is important to note that properly focusing the UV laser beam on an individual cell deep within the embryo is difficult. Sub-optimal focusing can lead to unintentional uncaging of cells outside the focal plane of the target cell. Also, uncaged Rhodamine has a short half-life, and must be imaged within 48 hours or the signal may be difficult to see. Similarly uncaged FITC is sometimes difficult to image later in development, and thus detection by immunostaining is often performed. Injected embryos must also be kept in the dark to avoid non-specific uncaging from ambient light.
Aside from chemical tracers, we can also lineage trace with GFP mRNA injection, over-express a protein of interest by injecting mRNA, knockdown expression by shRNA injection or mutant protein construct injection to see what cell types and tissues are affected during embryogenesis. Particularly mRNAs encoding histone tagged with green, cyan, yellow or red fluorescent proteins co-injected with mRNA for a membrane-bound fusion protein conjugated to another fluorophore, greatly enhance our ability to obtain high-resolution images of individual cell movements over time. Such microinjection experiments allow highly specific and selective cell manipulations superior to gross ablation experiments. Thus, specificity will facilitate the effective observation of injected cells and their neighbors and resultant deviations from normal development.
Fate mapping is therefore an extremely powerful tool for biologists, with new and improved tools constantly evolving to allow great resolution of what goes on during embryogenesis in various model organisms. Many genetic and chemical tools have been generated that allow long-term cell lineage tracing, bringing insight into the longevity of embryonic stem cells for various tissues. The ability to over-express and knockdown putative cell-fate patterning molecules and fluorescently label them, will also enhance our understanding of the extrinsic and intrinsic molecular cues required by various cell types during embryogenesis.
References
- Bertrand, Julien Y., Neil C. Chi, Buyung Santoso, Shutian Teng, Didier Y. R. Stainier, and David Traver. "Haematopoietic Stem cells Derive Directly From Aortic Endothelium During Development." Nature 464 (2010):108-11.
- Bertrand, Julien Y., Albert D. Kim, Emily P. Violette, David L. Stachura, Jennifer L. Cisson, and David Traver. "Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo." Development 134 (2007): 4147-56.
- Bowes JB, Snyder KA, Segerdell E, Jarabek CJ, Azam K, Zorn AM, Vize PD. (2009) Xenbase: gene expression and improved integration. Nucleic Acids Res., doi:10.1093/nar/gkp953. 3 April 2011. http://www.xenbase.org/anatomy/alldev.do
- Dale, L. and JMW Slack. "Fate map for the 32-cell stage of Xenopus laevis." Development 99 (1987): 527-51.
- Gilbert, Scott F. Developmental Biology. 6th Edition. Sunderland (MA): Sinauer Associates, 2000.
- Gimlich RL and Jochen Braun. "Improved fluorescent compounds for tracing cell lineage." Developmental Biology. 109 (1985):509-14.
- Hatta, Kohei, Hitomi Tsujii, and Tomomi Omura. "Cell tracking using a photoconvertible fluorescent protein." Nature Protocols 1 (2006): 960-7.
- Kuhn, Ralph, Frieder Schwenk, Michel Aguet, and Klaus Rajewsky. "Inducible Gene Targeting in Mice." Science 269 (1995):1427-9.
- Molecular Probes. Invitrogen Technologies. 6 March 2011 <http://www.invitrogen.com/site/us/en/home/brands/Molecular-Probes.html>
- Murayama, Emi, Karima Kissa, Agustin Zapata, Elodie Mordelet, Valerie Briolat, Hui-Feng Lin, Robert I. Handin and Philippe Herbomel. "Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development." Immunity 25 (2006): 963-75.
- Nieuwkoop and Faber (1994) Normal Table of Xenopus laevis (Daudin). Garland Publishing Inc, New York ISBN 0-8153-1896-0.
- Sternberg, Nat, and Daniel Hamilton. "Bacteriophage P1 Site-specific Recombination." Journal of Molecular Biology 150 (1981):467-86.
- Sulston, J.E., E. Schierenberg, J. G. White, and J.N. Thomson. "The Embryonic Cell Lineage of the Nematode Caenorhabditis elegans." Developmental Biology. 100 (1983): 64-119.
- Vogt, Walter. Gestaltungsanalyse am Amphibienkeim mit örtlicher Vitalfärbung. II. Teil Gastrulation und Mesodermbildung bei Urodelen und Anuren. Wilhelm Roux Arch. Entwicklungsmech. Org. 1929;120:384–706.
- Weisblat, David A., Roy T. Sawyer, and Gunther S. Stent. "Cell Lineage Analysis by Intracellular Injection of a Tracer Enzyme." Science 202 (1978):1295-8.
- Weisblat, David A., Saul L. Zackson, Seth S. Blair, and Janis D. Young. "Cell Lineage Analysis by Intracellular Injection of Fluorescent Tracers." Science 209 (1980): 1538-41.
External links
- http://worms.zoology.wisc.edu/frogs/gast/gast_fatemap.html
- Fate-Mapping Technique: Using Carbocyanine Dyes for Vital Labeling of Cells in Gastrula-Stage Mouse Embryos Cultured in Vitro