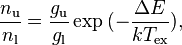Excitation temperature
From Wikipedia, the free encyclopedia
The Excitation Temperature (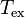 ) is defined for a population of particles via the Boltzmann factor. It satisfies
) is defined for a population of particles via the Boltzmann factor. It satisfies
where nu and nl represent the number of particles in an upper (e.g. excited) and lower (e.g. ground) state, and gu and gl their statistical weights respectively.
Thus the excitation temperature is the temperature at which we would expect to find a system with this ratio of level populations. However it has no actual physical meaning except when in local thermodynamical equilibrium. The excitation temperature can even be negative for a system with inverted levels (such as a maser).
In observations of the 21 cm line, the apparent value of the excitation temperature is often called the "spin temperature".[1]
References
- ↑ Dickey, J. M.; Mebold, U.; Stanimirovic, S.; Staveley‐Smith, L. (2000). "Cold Atomic Gas in the Small Magellanic Cloud". The Astrophysical Journal 536 (2): 756. doi:10.1086/308953.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.