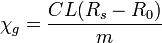Evans balance
An Evans balance is a device for measuring magnetic susceptibility. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field. Various practical devices are available for the measurement of susceptibility, which differ in the shape of the magnetic field and the way the force is measured.[1]
In the Gouy balance there is a homogeneous field in the central region between two (flat) poles of a permanent magnet, or an electromagnet. The sample, in the form of a powder in a cylindrical tube, is suspended in such a way the one end lies in the centre of the field and the other is effectively outside the magnetic field. The force is measured by an analytical balance
The Evans balance employs a similar sample configuration, but measures the force on the magnet.[2] Two pairs of magnets are placed at opposite ends of a beam making a balanced system with a magnetic field at each end. When a sample, which is fixed in a holder, is introduced into the field of one magnet, that magnet experiences a force which would deflect the beam. A magnetic field is generated at the second magnet which, by negative feedback, restores the beam to its original position. The current required to do this is proportional to the force exerted on the first magnet. The advantage of this system is that it is cheap to construct as it does not require a precision weighting device.
Calibration
The Evans balance measures susceptibility indirectly by referring to a calibration standard of known susceptibility. The most convenient compound for this purpose is mercury cobalt thiocyanate, HgCo(NCS)4, which has a susceptibility of 16.44×10−6 (±0.5%) CGI at 20°C.[3] Three readings of the meter are needed, of an empty tube, R0 of the tube filled with calibrant and of the tube filled with the sample, Rs. The first two provide a calibration constant, C. The mass susceptibility in grams is calculated as
where L is the length of the sample and m is its mass in grams. The reading for the empty tube is needed because the tube glass is diamagnetic.
References
- ↑ O'Connor, C.J. (1982). Lippard, S.J., ed. Magnetic susceptibility measurements. Progress in Inorganic Chemistry 29. Wiley. p. 203. ISBN 978-0-470-16680-2.
- ↑ Illustration of commercial Evans balance
- ↑ Figgis, B.N.; Lewis, J. (1960). "The Magnetochemistry of Complex Compounds". In Lewis. J. and Wilkins. R.G. Modern Coordination Chemistry. New York: Wiley. p. 415