Eutectic system

A eutectic system is a mixture of chemical compounds or elements that have a single chemical composition that solidifies at a lower temperature than any other composition made up of the same ingredients. This composition is known as the eutectic composition and the temperature at which it solidifies is known as the eutectic temperature.
On a phase diagram the intersection of the eutectic temperature and the eutectic composition gives the eutectic point.[1] Non-eutectic mixtures will display solidification of one component of the mixture before the other. Not all binary alloys have a eutectic point; for example, in the silver-gold system the melt temperature (liquidus) and freeze temperature (solidus) both increase monotonically as the mix changes from pure silver to pure gold.[2]
Eutectic reaction

The eutectic reaction is defined as follows:[3]
This type of reaction is an invariant reaction, because it is in thermal equilibrium; another way to define this is the Gibbs free energy equals zero. Tangibly, this means the liquid and two solid solutions all coexist at the same time and are in chemical equilibrium. There is also a thermal arrest for the duration of the change of phase during which the temperature of the system does not change.[3]
The resulting solid macrostructure from a eutectic reaction depends on a few factors. The most important factor is how the two solid solutions nucleate and grow. The most common structure is a lamellar structure, but other possible structures include rodlike, globular, and acicular.[4]
Non-eutectic compositions
Compositions of eutectic systems that are not the eutectic composition are called either hypoeutectic or hypereutectic. Hypoeutectic compositions are those to the left of the eutectic composition while hypereutectic compositions are those to the right. As the temperature of a non-eutectic composition is lowered the liquid mixture will precipitate one component of the mixture before the other.[3]
Types
Alloys
Eutectic alloys have two or more materials and have a eutectic composition. When a non-eutectic alloy solidifies, its components solidify at different temperatures, exhibiting a plastic melting range. Conversely, when a well-mixed, eutectic alloy melts, it does so at a single, sharp temperature. The various phase transformations that occur during the solidification of a particular alloy composition can be understood by drawing a vertical line from the liquid phase to the solid phase on the phase diagram for that alloy.
Some uses include:
- Eutectic alloys for soldering, composed of tin (Sn), lead (Pb) and sometimes silver (Ag) or gold (Au) — especially Sn63Pb37 alloy formula for electronics
- Casting alloys, such as aluminium-silicon and cast iron (at the composition of 4.3% carbon in iron producing an austenite-cementite eutectic)
- Silicon chips are bonded to gold-plated substrates through a silicon-gold eutectic by the application of ultrasonic energy to the chip. See eutectic bonding.
- Brazing, where diffusion can remove alloying elements from the joint, so that eutectic melting is only possible early in the brazing process
- Temperature response, e.g., Wood's metal and Field's metal for fire sprinklers
- Non-toxic mercury replacements, such as galinstan
- Experimental glassy metals, with extremely high strength and corrosion resistance
- Eutectic alloys of sodium and potassium (NaK) that are liquid at room temperature and used as coolant in experimental fast neutron nuclear reactors.
Others
- Sodium chloride and water form a eutectic mixture whose eutectic point is −21.2˚C[5] and 23.3% salt by mass.[6] The eutectic nature of salt and water is exploited when salt is spread on roads to aid snow removal, or mixed with ice to produce low temperatures (for example, in traditional ice cream making).
- Ethanol–water has an unusually biased eutectic point.
 Solid-liquid phase change of ethanol water mixtures
Solid-liquid phase change of ethanol water mixtures - "Solar salt", 60% NaNO3 and 40% KNO3, forms a eutectic molten salt mixture which is used for thermal energy storage in concentrated solar power plants.[7] To reduce the eutectic melting point in the solar molten salts calcium nitrate is used in the following proportion: 42% Ca(NO3)2, 43% KNO3, and 15% NaNO3.
- Lidocaine and prilocaine—both are solids at room temperature—form a eutectic that is an oil with a 16 °C (61 °F) melting point that is used in eutectic mixture of local anesthetic (EMLA) preparations.
- Menthol and camphor, both solids at room temperature, form a eutectic that is a liquid at room temperature in the following proportions: 8:2, 7:3, 6:4, and 5:5. Both substances are common ingredients in pharmacy extemporaneous preparations.[citation needed]
- Minerals may form eutectic mixtures in igneous rocks, giving rise to characteristic intergrowth textures exhibited by granophyre.[8]
- Some inks are eutectic mixtures, allowing inkjet printers to operate at lower temperatures.[9]
Other critical points
Eutectoid

When the solution above the transformation point is solid, rather than liquid, an analogous eutectoid transformation can occur. For instance, in the iron-carbon system, the austenite phase can undergo a eutectoid transformation to produce ferrite and cementite, often in lamellar structures such as pearlite and bainite. This eutectoid point occurs at 727 °C (1,341 °F) and about 0.76% carbon.[10]
Peritectoid
A peritectoid transformation is a type of isothermal reversible reaction that have two solid phases reacting with each other upon cooling of a binary, ternary, ..., 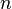 alloy to create a completely different and single solid phase.[11] The reaction plays a key role in the order and decomposition of quasicrystalline phases in several alloy types.[12]
alloy to create a completely different and single solid phase.[11] The reaction plays a key role in the order and decomposition of quasicrystalline phases in several alloy types.[12]
Peritectic
Peritectic transformations are also similar to eutectic reactions. Here, a liquid and solid phase of fixed proportions react at a fixed temperature to yield a single solid phase. Since the solid product forms at the interface between the two reactants, it can form a diffusion barrier and generally causes such reactions to proceed much more slowly than eutectic or eutectoid transformations. Because of this, when a peritectic composition solidifies it does not show the lamellar structure that is found with eutectic solidification.
Such a transformation exists in the iron-carbon system, as seen near the upper-left corner of the figure. It resembles an inverted eutectic, with the δ phase combining with the liquid to produce pure austenite at 1,495 °C (2,723 °F) and 0.17% carbon.

Peritectic decomposition. Up to this point in the discussion transformations have been addressed from the point of view of cooling. They also can be discussed noting the changes that occur to some solid chemical compounds as they are heated. Rather than melting, at the peritectic decomposition temperature, the compound decomposes into another solid compound and a liquid. The proportion of each is determined by the lever rule. The vocabulary changes slightly. Just as the cooling of water, which leads to ice, is termed freezing, the warming of ice leads to melting. In the Al-Au phase diagram, for example, it can be seen that only two of the phases melt congruently, AuAl2 and Au2Al. The rest peritectically decompose.
Eutectic calculation
The composition and temperature of a eutectic can be calculated from enthalpy and entropy of fusion of each components.[13]
The free Gibbs enthalpy G depends on its own differential by Eq.
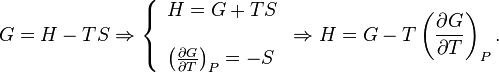
Thus, the G/T derivative at constant pressure is calculated by equation Eq.

The chemical potential  is calculated if we assume the activity is equal to the
concentration we suppose the activity equal to the concentration.
is calculated if we assume the activity is equal to the
concentration we suppose the activity equal to the concentration.
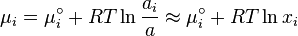
At the equilibrium, 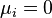 , thus
, thus 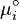 is obtained by:
is obtained by:

Using and integrating gives Eq.
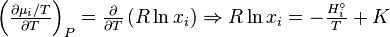
The integration constant K may be determined for a pure
component with a melting temperature 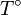 and an enthalpy of
fusion
and an enthalpy of
fusion 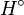 Eq.
Eq.

We obtain a relation that determines the molar fraction as a function of the temperature for each component.
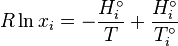
The mixture of n components is described by the system


that can be solved by
![{\begin{array}{c}\left[{{{\begin{array}{*{20}c}{\Delta x_{1}}\\{\Delta x_{2}}\\{\Delta x_{3}}\\\vdots \\{\Delta x_{{n-1}}}\\{\Delta T}\\\end{array}}}}\right]=\left[{{{\begin{array}{*{20}c}{1/x_{1}}&0&0&0&0&{-{\frac {H_{1}^{\circ }}{RT^{{2}}}}}\\0&{1/x_{2}}&0&0&0&{-{\frac {H_{2}^{\circ }}{RT^{{2}}}}}\\0&0&{1/x_{3}}&0&0&{-{\frac {H_{3}^{\circ }}{RT^{{2}}}}}\\0&0&0&\ddots &0&{-{\frac {H_{4}^{\circ }}{RT^{{2}}}}}\\0&0&0&0&{1/x_{{n-1}}}&{-{\frac {H_{{n-1}}^{\circ }}{RT^{{2}}}}}\\{{\frac {-1}{1-\sum \limits _{{1=1}}^{{n-1}}{x_{i}}}}}&{{\frac {-1}{1-\sum \limits _{{1=1}}^{{n-1}}{x_{i}}}}}&{{\frac {-1}{1-\sum \limits _{{1=1}}^{{n-1}}{x_{i}}}}}&{{\frac {-1}{1-\sum \limits _{{1=1}}^{{n-1}}{x_{i}}}}}&{{\frac {-1}{1-\sum \limits _{{1=1}}^{{n-1}}{x_{i}}}}}&{-{\frac {H_{n}^{\circ }}{RT^{{2}}}}}\\\end{array}}}}\right]^{{-1}}.\left[{{{\begin{array}{*{20}c}{\ln x_{1}+{\frac {H_{1}^{\circ }}{RT}}-{\frac {H_{1}^{\circ }}{RT_{1}^{\circ }}}}\\{\ln x_{2}+{\frac {H_{2}^{\circ }}{RT}}-{\frac {H_{2}^{\circ }}{RT_{2}^{\circ }}}}\\{\ln x_{3}+{\frac {H_{3}^{\circ }}{RT}}-{\frac {H_{3}^{\circ }}{RT_{3}^{\circ }}}}\\\vdots \\{\ln x_{{n-1}}+{\frac {H_{{n-1}}^{\circ }}{RT}}-{\frac {H_{{n-1}}^{\circ }}{RT_{{n-1i}}^{\circ }}}}\\{\ln \left({1-\sum \limits _{{i=1}}^{{n-1}}{x_{i}}}\right)+{\frac {H_{n}^{\circ }}{RT}}-{\frac {H_{n}^{\circ }}{RT_{n}^{\circ }}}}\\\end{array}}}}\right]\end{array}}](/2014-wikipedia_en_all_02_2014/I/media/0/6/7/c/067cd8690de26634bcab9cfd0339759d.png)
See also
- Azeotrope, constant boiling mixture
- Freezing-point depression
References
- ↑ Smith & Hashemi 2006, pp. 326–327.
- ↑ http://www.crct.polymtl.ca/fact/phase_diagram.php?file=Ag-Au.jpg&dir=SGTE
- ↑ 3.0 3.1 3.2 Smith & Hashemi 2006, p. 327.
- ↑ Smith & Hashemi 2006, pp. 332–333.
- ↑ Muldrew, Ken; Locksley E. McGann (1997). "Phase Diagrams". Cryobiology—A Short Course. University of Calgary. Retrieved 2006-04-29.
- ↑ Senese, Fred (1999). "Does salt water expand as much as fresh water does when it freezes?". Solutions: Frequently asked questions. Department of Chemistry, Frostburg State University. Retrieved 2006-04-29.
- ↑ "Molten salts properties". Archimede Solar Plant Specs.
- ↑ Fichter, Lynn S. (2000). "Igneous Phase Diagrams". Igneous Rocks. James Madison University. Retrieved 2006-04-29.
- ↑ Davies, Nicholas A.; Beatrice M. Nicholas (1992). "Eutectic compositions for hot melt jet inks". US Patent & Trademark Office, Patent Full Text and Image Database. United States Patent and Trademark Office. Retrieved 2006-04-29.
- ↑ Iron-Iron Carbide Phase Diagram Example
- ↑ IUPAC Compendium of Chemical Terminology, Electronic version. "Peritectoid Reaction" Retrieved May 22, 2007.
- ↑ Numerical Model of Peritectoid Transformation. Peritectoid Transformation Retrieved May 22, 2007.
- ↑ International Journal of Modern Physics C, Vol. 15, No. 5. (2004), pp. 675-687
Bibliography
- Smith, William F.; Hashemi, Javad (2006), Foundations of Materials Science and Engineering (4th ed.), McGraw-Hill, ISBN 0-07-295358-6.
Further reading
| Look up eutectic in Wiktionary, the free dictionary. |
- Askeland, Donald R.; Pradeep P. Phule (2005). The Science and Engineering of Materials. Thomson-Engineering. ISBN 0-534-55396-6.
- Easterling, Edward (1992). Phase Transformations in Metals and Alloys. CRC. ISBN 0-7487-5741-4.
- Mortimer, Robert G. (2000). Physical Chemistry. Academic Press. ISBN 0-12-508345-9.
- Reed-Hill, R.E.; Reza Abbaschian (1992). Physical Metallurgy Principles. Thomson-Engineering. ISBN 0-534-92173-6.
- Sadoway, Donald (2004). "Phase Equilibria and Phase Diagrams" (pdf). 3.091 Introduction to Solid State Chemistry, Fall 2004. MIT Open Courseware. Archived from the original on 2005-10-20. Retrieved 2006-04-12.
![{\text{Liquid}}{\xrightarrow[ {{\text{cooling}}}]{{\text{eutectic temperature}}}}\alpha \,\,{\text{solid solution}}+\beta \,\,{\text{solid solution}}](/2014-wikipedia_en_all_02_2014/I/media/1/f/6/e/1f6e610faa6afd06bf7e16c7bb620167.png)