Ethanol fuel
| Renewable energy |
|---|
Ethanol fuel is ethanol (ethyl alcohol), the same type of alcohol found in alcoholic beverages. It is most often used as a motor fuel, mainly as a biofuel additive for gasoline. World ethanol production for transport fuel tripled between 2000 and 2007 from 17 billion to more than 52 billion liters. From 2007 to 2008, the share of ethanol in global gasoline type fuel use increased from 3.7% to 5.4%.[2] In 2011 worldwide ethanol fuel production reached 22.36 billion U.S. liquid gallons (bg) (84.6 billion liters), with the United States as the top producer with 13.9 bg (52.6 billion liters), accounting for 62.2% of global production, followed by Brazil with 5.6 bg (21.1 billion liters).[3] Ethanol fuel has a "gasoline gallon equivalency" (GGE) value of 1.5 US gallons (5.7 L), which means 1.5 gallons of ethanol produce the energy of one gallon of gasoline.[4]
Ethanol fuel is widely used in Brazil and in the United States, and together both countries were responsible for 87.1% of the world's ethanol fuel production in 2011.[3] Most cars on the road today in the U.S. can run on blends of up to 10% ethanol,[5] and ethanol represented 10% of the U.S. gasoline fuel supply derived from domestic sources in 2011.[3] Since 1976 the Brazilian government has made it mandatory to blend ethanol with gasoline, and since 2007 the legal blend is around 25% ethanol and 75% gasoline (E25).[6] By December 2011 Brazil had a fleet of 14.8 million flex-fuel automobiles and light trucks[7][8] and 1.5 million flex-fuel motorcycles[9][10][11] that regularly use neat ethanol fuel (known as E100).
Bioethanol is a form of quasi-renewable energy that can be produced from agricultural feedstocks. It can be made from very common crops such as sugar cane, potato, manioc and corn. There has been considerable debate about how useful bioethanol will be in replacing gasoline. Concerns about its production and use relate to increased food prices due to the large amount of arable land required for crops,[12] as well as the energy and pollution balance of the whole cycle of ethanol production, especially from corn.[13][14] Recent developments with cellulosic ethanol production and commercialization may allay some of these concerns.[15]
Cellulosic ethanol offers promise because cellulose fibers, a major and universal component in plant cells walls, can be used to produce ethanol.[16][17] According to the International Energy Agency, cellulosic ethanol could allow ethanol fuels to play a much bigger role in the future than previously thought.[18]
Chemistry
During ethanol fermentation, glucose and other sugars in the corn (or sugarcane or other crops) are converted into ethanol and carbon dioxide.
- C6H12O6 → 2 C2H5OH+ 2 CO2 + heat
Like any fermentation reaction, the fermentation is not 100% selective, and other side products such acetic acid, glycols and many other products are formed to a considerable extent and need to be removed during the purification of the ethanol. The fermentation takes place in aqueous solution and the resulting solution after fermentation has an ethanol content of around 15%. The ethanol is subsequently isolated and purified by a combination of adsorption and distillation techniques. The purification is very energy intensive.
During combustion ethanol reacts with oxygen to produce carbon dioxide, water, and heat:
- C2H5OH + 3 O2 → 2 CO2 + 3 H2O + heat
Starch and cellulose are molecules that are strings of glucose molecules. It is also possible to generate ethanol out of cellulosic materials. However, a pretreatment is necessary that splits the cellulose into glycose molecules and other sugars which subsequently can be fermented. The resulting product is called cellulosic ethanol, indicating its source.
Ethanol may also be produced industrially from ethene (ethylene), by hydrolysis of the double bond in the presence of catalysts and high temperature.
- C2H4 + H2O → C2H5OH
The by far largest fraction of the global ethanol production, however, is produced by fermentation
Sources



Ethanol is a quasi-renewable energy source because while the energy is partially generated by using a resource, sunlight, which cannot be depleted, the harvesting process requires vast amounts of energy that typically comes from non-renewable sources.[19] Creation of ethanol starts with photosynthesis causing a feedstock, such as sugar cane or a grain such as maize (corn), to grow. These feedstocks are processed into ethanol.
About 5% of the ethanol produced in the world in 2003 was actually a petroleum product.[20] It is made by the catalytic hydration of ethylene with sulfuric acid as the catalyst. It can also be obtained via ethylene or acetylene, from calcium carbide, coal, oil gas, and other sources. Two million tons of petroleum-derived ethanol are produced annually. The principal suppliers are plants in the United States, Europe, and South Africa.[21] Petroleum derived ethanol (synthetic ethanol) is chemically identical to bio-ethanol and can be differentiated only by radiocarbon dating.[22]
Bio-ethanol is usually obtained from the conversion of carbon based feedstock. Agricultural feedstocks are considered renewable because they get energy from the sun using photosynthesis, provided that all minerals required for growth (such as nitrogen and phosphorus) are returned to the land. Ethanol can be produced from a variety of feedstocks such as sugar cane, bagasse, miscanthus, sugar beet, sorghum, grain, switchgrass, barley, hemp, kenaf, potatoes, sweet potatoes, cassava, sunflower, fruit, molasses, corn, stover, grain, wheat, straw, cotton, other biomass, as well as many types of cellulose waste and harvestings, whichever has the best well-to-wheel assessment.
An alternative process to produce bio-ethanol from algae is being developed by the company Algenol. Rather than grow algae and then harvest and ferment it, the algae grow in sunlight and produce ethanol directly which is removed without killing the algae. It is claimed the process can produce 6,000 US gallons per acre (56,000 litres per ha) per year compared with 400 US gallons per acre (3,750 l/ha) for corn production.[23]
Currently, the first generation processes for the production of ethanol from corn use only a small part of the corn plant: the corn kernels are taken from the corn plant and only the starch, which represents about 50% of the dry kernel mass, is transformed into ethanol. Two types of second generation processes are under development. The first type uses enzymes and yeast fermentation to convert the plant cellulose into ethanol while the second type uses pyrolysis to convert the whole plant to either a liquid bio-oil or a syngas. Second generation processes can also be used with plants such as grasses, wood or agricultural waste material such as straw.
Production process
The basic steps for large scale production of ethanol are: microbial (yeast) fermentation of sugars, distillation, dehydration (requirements vary, see Ethanol fuel mixtures, below), and denaturing (optional). Prior to fermentation, some crops require saccharification or hydrolysis of carbohydrates such as cellulose and starch into sugars. Saccharification of cellulose is called cellulolysis (see cellulosic ethanol). Enzymes are used to convert starch into sugar.[24]
Fermentation
Ethanol is produced by microbial fermentation of the sugar. Microbial fermentation will currently only work directly with sugars. Two major components of plants, starch and cellulose, are both made up of sugars, and can in principle be converted to sugars for fermentation. Currently, only the sugar (e.g. sugar cane) and starch (e.g. corn) portions can be economically converted. There is much activity in the area of cellulosic ethanol, where the cellulose part of a plant is broken down to sugars and subsequently converted to ethanol.
Distillation


For the ethanol to be usable as a fuel, the majority of the water must be removed. Most of the water is removed by distillation, but the purity is limited to 95–96% due to the formation of a low-boiling water-ethanol azeotrope with maximum (95.6% m/m (96.5% v/v) ethanol and 4.4% m/m (3.5% v/v) water). This mixture is called hydrous ethanol and can be used as a fuel alone, but unlike anhydrous ethanol, hydrous ethanol is not miscible in all ratios with gasoline, so the water fraction is typically removed in further treatment in order to burn in combination with gasoline in gasoline engines.[25]
Dehydration
There are basically three dehydration processes to remove the water from an azeotropic ethanol/water mixture. The first process, used in many early fuel ethanol plants, is called azeotropic distillation and consists of adding benzene or cyclohexane to the mixture. When these components are added to the mixture, it forms a heterogeneous azeotropic mixture in vapor-liquid-liquid equilibrium, which when distilled produces anhydrous ethanol in the column bottom, and a vapor mixture of water, ethanol, and cyclohexane/benzene. When condensed, this becomes a two-phase liquid mixture. The heavier phase, poor in the entrainer (benzene or cyclohexane), is stripped of the entrainer and recycled to the feed, while the lighter phase together with condensate from the stripping is recycled to the second column. Another early method, called extractive distillation, consists of adding a ternary component which will increase ethanol's relative volatility. When the ternary mixture is distilled, it will produce anhydrous ethanol on the top stream of the column.
With increasing attention being paid to saving energy, many methods have been proposed that avoid distillation altogether for dehydration. Of these methods, a third method has emerged and has been adopted by the majority of modern ethanol plants. This new process uses molecular sieves to remove water from fuel ethanol. In this process, ethanol vapor under pressure passes through a bed of molecular sieve beads. The bead's pores are sized to allow absorption of water while excluding ethanol. After a period of time, the bed is regenerated under vacuum or in the flow of inert atmosphere (e.g. N2) to remove the absorbed water. Two beds are often used so that one is available to absorb water while the other is being regenerated. This dehydration technology can account for energy saving of 3,000 btus/gallon (840 kJ/L) compared to earlier azeotropic distillation.[26]
Technology
Ethanol-based engines
Ethanol is most commonly used to power automobiles, though it may be used to power other vehicles, such as farm tractors, boats and airplanes. Ethanol (E100) consumption in an engine is approximately 51% higher than for gasoline since the energy per unit volume of ethanol is 34% lower than for gasoline.[27][28] The higher compression ratios in an ethanol-only engine allow for increased power output and better fuel economy than could be obtained with lower compression ratios.[29][30] In general, ethanol-only engines are tuned to give slightly better power and torque output than gasoline-powered engines. In flexible fuel vehicles, the lower compression ratio requires tunings that give the same output when using either gasoline or hydrated ethanol. For maximum use of ethanol's benefits, a much higher compression ratio should be used.[31] Current high compression neat ethanol engine designs are approximately 20 to 30% less fuel efficient than their gasoline-only counterparts.[32]
Ethanol contains soluble and insoluble contaminants.[33] These soluble contaminants, halide ions such as chloride ions, have a large effect on the corrosivity of alcohol fuels. Halide ions increase corrosion in two ways; they chemically attack passivating oxide films on several metals causing pitting corrosion, and they increase the conductivity of the fuel. Increased electrical conductivity promotes electric, galvanic, and ordinary corrosion in the fuel system. Soluble contaminants, such as aluminum hydroxide, itself a product of corrosion by halide ions, clog the fuel system over time.
Ethanol is hygroscopic, meaning it will absorb water vapor directly from the atmosphere. Because absorbed water dilutes the fuel value of the ethanol (although it suppresses engine knock) and may cause phase separation of ethanol-gasoline blends, containers of ethanol fuels must be kept tightly sealed. This high miscibility with water means that ethanol cannot be efficiently shipped through modern pipelines, like liquid hydrocarbons, over long distances.[34] Mechanics also have seen increased cases of damage to small engines, in particular, the carburetor, attributable to the increased water retention by ethanol in fuel.[35]
A 2004 MIT study[36] and an earlier paper published by the Society of Automotive Engineers[37] identify a method to exploit the characteristics of fuel ethanol substantially better than mixing it with gasoline. The method presents the possibility of leveraging the use of alcohol to achieve definite improvement over the cost-effectiveness of hybrid electric. The improvement consists of using dual-fuel direct-injection of pure alcohol (or the azeotrope or E85) and gasoline, in any ratio up to 100% of either, in a turbocharged, high compression-ratio, small-displacement engine having performance similar to an engine having twice the displacement. Each fuel is carried separately, with a much smaller tank for alcohol. The high-compression (which increases efficiency) engine will run on ordinary gasoline under low-power cruise conditions. Alcohol is directly injected into the cylinders (and the gasoline injection simultaneously reduced) only when necessary to suppress ‘knock’ such as when significantly accelerating. Direct cylinder injection raises the already high octane rating of ethanol up to an effective 130. The calculated over-all reduction of gasoline use and CO2 emission is 30%. The consumer cost payback time shows a 4:1 improvement over turbo-diesel and a 5:1 improvement over hybrid. The problems of water absorption into pre-mixed gasoline (causing phase separation), supply issues of multiple mix ratios and cold-weather starting are also avoided.
Ethanol's higher octane rating allows an increase of an engine's compression ratio for increased thermal efficiency.[29] In one study, complex engine controls and increased exhaust gas recirculation allowed a compression ratio of 19.5 with fuels ranging from neat ethanol to E50. Thermal efficiency up to approximately that for a diesel was achieved.[38] This would result in the fuel economy of a neat ethanol vehicle to be about the same as one burning gasoline.
Since 1989 there have also been ethanol engines based on the diesel principle operating in Sweden.[39] They are used primarily in city buses, but also in distribution trucks and waste collectors. The engines, made by Scania, have a modified compression ratio, and the fuel (known as ED95) used is a mix of 93.6% ethanol and 3.6% ignition improver, and 2.8% denaturants.[40] The ignition improver makes it possible for the fuel to ignite in the diesel combustion cycle. It is then also possible to use the energy efficiency of the diesel principle with ethanol. These engines have been used in the United Kingdom by Reading Transport but the use of bioethanol fuel is now being phased out.
Engine cold start during the winter

High ethanol blends present a problem to achieve enough vapor pressure for the fuel to evaporate and spark the ignition during cold weather (since ethanol tends to increase fuel enthalpy of vaporization[41]). When vapor pressure is below 45 kPa starting a cold engine becomes difficult.[42] In order to avoid this problem at temperatures below 11 °C (52 °F)), and to reduce ethanol higher emissions during cold weather, both the US and the European markets adopted E85 as the maximum blend to be used in their flexible fuel vehicles, and they are optimized to run at such a blend. At places with harsh cold weather, the ethanol blend in the US has a seasonal reduction to E70 for these very cold regions, though it is still sold as E85.[43][44] At places where temperatures fall below −12 °C (10 °F) during the winter, it is recommended to install an engine heater system, both for gasoline and E85 vehicles. Sweden has a similar seasonal reduction, but the ethanol content in the blend is reduced to E75 during the winter months.[44][45]
Brazilian flex fuel vehicles can operate with ethanol mixtures up to E100, which is hydrous ethanol (with up to 4% water), which causes vapor pressure to drop faster as compared to E85 vehicles. As a result, Brazilian flex vehicles are built with a small secondary gasoline reservoir located near the engine. During a cold start pure gasoline is injected to avoid starting problems at low temperatures. This provision is particularly necessary for users of Brazil's southern and central regions, where temperatures normally drop below 15 °C (59 °F) during the winter. An improved flex engine generation was launched in 2009 that eliminates the need for the secondary gas storage tank.[46][47] In March 2009 Volkswagen do Brasil launched the Polo E-Flex, the first Brazilian flex fuel model without an auxiliary tank for cold start.[48][49]
Ethanol fuel mixtures

To avoid engine stall due to "slugs" of water in the fuel lines interrupting fuel flow, the fuel must exist as a single phase. The fraction of water that an ethanol-gasoline fuel can contain without phase separation increases with the percentage of ethanol.[50] This shows, for example, that E30 can have up to about 2% water. If there is more than about 71% ethanol, the remainder can be any proportion of water or gasoline and phase separation will not occur. The fuel mileage declines with increased water content. The increased solubility of water with higher ethanol content permits E30 and hydrated ethanol to be put in the same tank since any combination of them always results in a single phase. Somewhat less water is tolerated at lower temperatures. For E10 it is about 0.5% v/v at 70 F and decreases to about 0.23% v/v at −30 F.[51]

In many countries cars are mandated to run on mixtures of ethanol. All Brazilian light-duty vehicles are built to operate for an ethanol blend of up to 25% (E25), and since 1993 a federal law requires mixtures between 22% and 25% ethanol, with 25% required as of mid July 2011.[52] In the United States all light-duty vehicles are built to operate normally with an ethanol blend of 10% (E10). At the end of 2010 over 90 percent of all gasoline sold in the U.S. was blended with ethanol.[53] In January 2011 the U.S. Environmental Protection Agency (EPA) issued a waiver to authorize up to 15% of ethanol blended with gasoline (E15) to be sold only for cars and light pickup trucks with a model year of 2001 or newer.[54][55] Other countries have adopted their own requirements.
Beginning with the model year 1999, an increasing number of vehicles in the world are manufactured with engines which can run on any fuel from 0% ethanol up to 100% ethanol without modification. Many cars and light trucks (a class containing minivans, SUVs and pickup trucks) are designed to be flexible-fuel vehicles using ethanol blends up to 85% (E85) in North America and Europe, and up to 100% (E100) in Brazil. In older model years, their engine systems contained alcohol sensors in the fuel and/or oxygen sensors in the exhaust that provide input to the engine control computer to adjust the fuel injection to achieve stochiometric (no residual fuel or free oxygen in the exhaust) air-to-fuel ratio for any fuel mix. In newer models, the alcohol sensors have been removed, with the computer using only oxygen and airflow sensor feedback to estimate alcohol content. The engine control computer can also adjust (advance) the ignition timing to achieve a higher output without pre-ignition when it predicts that higher alcohol percentages are present in the fuel being burned. This method is backed up by advanced knock sensors – used in most high performance gasoline engines regardless of whether they are designed to use ethanol or not – that detect pre-ignition and detonation.
Hydrous ethanol corrosion
High alcohol fuel blends are reputed to cause corrosion of aluminum fuel system components. However, studies indicate that the addition of water to the high alcohol fuel blends helps prevent corrosion. This is shown in SAE paper 2005-01-3708 Appendix 1.2 where gasoline/alcohol blends of E50, nP50,IP50 nB50, IB50 were tested on steel, copper, nickel, zinc, tin and three types of aluminum. The tests showed that when the water content was increased from 2000ppm to 1%, corrosion was no longer evident except some materials showed discolouration.
Fuel economy
In theory, all fuel-driven vehicles have a fuel economy (measured as miles per US gallon, or liters per 100 km) that is directly proportional to the fuel's energy content.[56] In reality, there are many other variables that come into play that affect the performance of a particular fuel in a particular engine. Ethanol contains approx. 34% less energy per unit volume than gasoline, and therefore in theory, burning pure ethanol in a vehicle will result in a 34% reduction in miles per US gallon, given the same fuel economy, compared to burning pure gasoline. Since ethanol has a higher octane rating, the engine can be made more efficient by raising its compression ratio. In fact using a variable turbocharger, the compression ratio can be optimized for the fuel being used, making fuel economy almost constant for any blend.[27][28] For E10 (10% ethanol and 90% gasoline), the effect is small (~3%) when compared to conventional gasoline,[57] and even smaller (1–2%) when compared to oxygenated and reformulated blends.[58] For E85 (85% ethanol), the effect becomes significant. E85 will produce lower mileage than gasoline, and will require more frequent refueling. Actual performance may vary depending on the vehicle. Based on EPA tests for all 2006 E85 models, the average fuel economy for E85 vehicles resulted 25.56% lower than unleaded gasoline.[59] The EPA-rated mileage of current USA flex-fuel vehicles[60] should be considered when making price comparisons, but E85 is a high performance fuel, with an octane rating of about 94–96, and should be compared to premium.[61] In one estimate[62] the US retail price for E85 ethanol is 2.62 US dollar per gallon or 3.71-dollar corrected for energy equivalency compared to a gallon of gasoline priced at 3.03-dollar. Brazilian cane ethanol (100%) is priced at 3.88-dollar against 4.91-dollar for E25 (as July 2007).
Consumer production systems
While biodiesel production systems have been marketed to home and business users for many years, commercialized ethanol production systems designed for end-consumer use have lagged in the marketplace. In 2008, two different companies announced home-scale ethanol production systems. The AFS125 Advanced Fuel System[63] from Allard Research and Development is capable of producing both ethanol and biodiesel in one machine, while the E-100 MicroFueler[64] from E-Fuel Corporation is dedicated to ethanol only.
Experience by country
The world's top ethanol fuel producers in 2011 were the United States with 13.9 billion U.S. liquid gallons (bg) (52.6 billion liters) and Brazil with 5.6 bg (21.1 billion liters), accounting together for 87.1% of world production of 22.36 billion US gallons (84.6 billion liters).[3] Strong incentives, coupled with other industry development initiatives, are giving rise to fledgling ethanol industries in countries such as Germany, Spain, France, Sweden, China, Thailand, Canada, Colombia, India, Australia, and some Central American countries.
| Annual Fuel Ethanol Production by Country (2007–2011)[3][65][66][67] Top 10 countries/regional blocks (Millions of U.S. liquid gallons per year) | ||||||
|---|---|---|---|---|---|---|
| World rank | Country/Region | 2011 | 2010 | 2009 | 2008 | 2007 |
| 1 | 13,900.00 | 13,231.00 | 10,938.00 | 9,235.00 | 6,485.00 | |
| 2 | 5,573.24 | 6,921.54 | 6,577.89 | 6,472.20 | 5,019.20 | |
| 3 | 1,199.31 | 1,176.88 | 1,039.52 | 733.60 | 570.30 | |
| 4 | 554.76 | 541.55 | 541.55 | 501.90 | 486.00 | |
| 5 | 435.20 | 89.80 | 79.20 | |||
| 6 | 462.30 | 356.63 | 290.59 | 237.70 | 211.30 | |
| 7 | 91.67 | 66.00 | 52.80 | |||
| 8 | 83.21 | 79.30 | 74.90 | |||
| 9 | 87.20 | 66.04 | 56.80 | 26.40 | 26.40 | |
| 10 | Other | 247.27 | ||||
| World Total | 22,356.09 | 22,946.87 | 19,534.99 | 17,335.20 | 13,101.70 | |
Environment
Energy balance
| Country | Type | Energy balance |
|---|---|---|
| United States | Corn ethanol | 1.3 |
| Brazil | Sugarcane ethanol | 8 |
| Germany | Biodiesel | 2.5 |
| United States | Cellulosic ethanol† | 2–36†† |
† experimental, not in commercial production
†† depending on production method
All biomass goes through at least some of these steps: it needs to be grown, collected, dried, fermented, and burned. All of these steps require resources and an infrastructure. The total amount of energy input into the process compared to the energy released by burning the resulting ethanol fuel is known as the energy balance (or "energy returned on energy invested"). Figures compiled in a 2007 by National Geographic Magazine[62] point to modest results for corn ethanol produced in the US: one unit of fossil-fuel energy is required to create 1.3 energy units from the resulting ethanol. The energy balance for sugarcane ethanol produced in Brazil is more favorable, with one unit of fossil-fuel energy required to create 8 from the ethanol. Energy balance estimates are not easily produced, thus numerous such reports have been generated that are contradictory. For instance, a separate survey reports that production of ethanol from sugarcane, which requires a tropical climate to grow productively, returns from 8 to 9 units of energy for each unit expended, as compared to corn which only returns about 1.34 units of fuel energy for each unit of energy expended.[68] A 2006 University of California Berkeley study, after analyzing six separate studies, concluded that producing ethanol from corn uses much less petroleum than producing gasoline.[69]
Carbon dioxide, a greenhouse gas, is emitted during fermentation and combustion. This is canceled out by the greater uptake of carbon dioxide by the plants as they grow to produce the biomass.[70] When compared to gasoline, depending on the production method, ethanol releases less greenhouse gases.[71][72]
Air pollution
Compared with conventional unleaded gasoline, ethanol is a particulate-free burning fuel source that combusts with oxygen to form carbon dioxide, water and aldehydes. The Clean Air Act requires the addition of oxygenates to reduce carbon monoxide emissions in the United States. The additive MTBE is currently being phased out due to ground water contamination, hence ethanol becomes an attractive alternative additive. Current production methods include air pollution from the manufacturer of macronutrient fertilizers such as ammonia.
A study by atmospheric scientists at Stanford University found that E85 fuel would increase the risk of air pollution deaths relative to gasoline by 9% in Los Angeles, USA: a very large, urban, car-based metropolis that is a worst case scenario.[73] Ozone levels are significantly increased, thereby increasing photochemical smog and aggravating medical problems such as asthma.[74][75]
Manufacture
In 2002, monitoring the process of ethanol production from corn revealed that they released VOCs (volatile organic compounds) at a higher rate than had previously been disclosed.[76] The U.S. Environmental Protection Agency (EPA) subsequently reached settlement with Archer Daniels Midland and Cargill, two of the largest producers of ethanol, to reduce emission of these VOCs. VOCs are produced when fermented corn mash is dried for sale as a supplement for livestock feed. Devices known as thermal oxidizers or catalytic oxidizers can be attached to the plants to burn off the hazardous gases.
Carbon dioxide
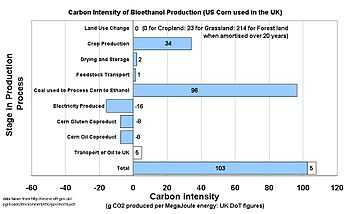

The calculation of exactly how much carbon dioxide is produced in the manufacture of bioethanol is a complex and inexact process, and is highly dependent on the method by which the ethanol is produced and the assumptions made in the calculation. A calculation should include:
- The cost of growing the feedstock
- The cost of transporting the feedstock to the factory
- The cost of processing the feedstock into bioethanol
Such a calculation may or may not consider the following effects:
- The cost of the change in land use of the area where the fuel feedstock is grown.
- The cost of transportation of the bioethanol from the factory to its point of use
- The efficiency of the bioethanol compared with standard gasoline
- The amount of Carbon Dioxide produced at the tail pipe.
- The benefits due to the production of useful bi-products, such as cattle feed or electricity.
The graph on the right shows figures calculated by the UK government for the purposes of the Renewable transport fuel obligation.[1]
The January 2006 Science article from UC Berkeley's ERG, estimated reduction from corn ethanol in GHG to be 13% after reviewing a large number of studies. In a correction to that article released shortly after publication, they reduce the estimated value to 7.4%. A National Geographic Magazine overview article (2007)[62] puts the figures at 22% less CO2 emissions in production and use for corn ethanol compared to gasoline and a 56% reduction for cane ethanol. Carmaker Ford reports a 70% reduction in CO2 emissions with bioethanol compared to petrol for one of their flexible-fuel vehicles.[77]
An additional complication is that production requires tilling new soil[78] which produces a one-off release of GHG that it can take decades or centuries of production reductions in GHG emissions to equalize.[79] As an example, converting grass lands to corn production for ethanol takes about a century of annual savings to make up for the GHG released from the initial tilling.[78]
Change in land use
Large-scale farming is necessary to produce agricultural alcohol and this requires substantial amounts of cultivated land. University of Minnesota researchers report that if all corn grown in the U.S. were used to make ethanol it would displace 12% of current U.S. gasoline consumption.[80] There are claims that land for ethanol production is acquired through deforestation, while others have observed that areas currently supporting forests are usually not suitable for growing crops.[81][82] In any case, farming may involve a decline in soil fertility due to reduction of organic matter,[83] a decrease in water availability and quality, an increase in the use of pesticides and fertilizers, and potential dislocation of local communities.[84] New technology enables farmers and processors to increasingly produce the same output using less inputs.[80]
Cellulosic ethanol production is a new approach which may alleviate land use and related concerns. Cellulosic ethanol can be produced from any plant material, potentially doubling yields, in an effort to minimize conflict between food needs vs. fuel needs. Instead of utilizing only the starch by-products from grinding wheat and other crops, cellulosic ethanol production maximizes the use of all plant materials, including gluten. This approach would have a smaller carbon footprint because the amount of energy-intensive fertilisers and fungicides remain the same for higher output of usable material. The technology for producing cellulosic ethanol is currently in the commercialization stage.[17][18]
Using biomass for electricity instead of ethanol
Converting biomass to electricity for charging electric vehicles may be a more "climate-friendly" transportation option than using biomass to produce ethanol fuel, according to an analysis published in Science in May 2009[85] "You make more efficient use of the land and more efficient use of the plant biomass by making electricity rather than ethanol," said Elliott Campbell, an environmental scientist at the University of California at Merced, who led the research. "It's another reason that, rather than race to liquid biofuels, we should consider other uses of bio-resources".
For bioenergy to become a widespread climate solution, technological breakthroughs are necessary, analysts say. Researchers continue to search for more cost-effective developments in both cellulosic ethanol and advanced vehicle batteries.[86]
Health costs of ethanol emissions
For each billion ethanol-equivalent gallons of fuel produced and combusted in the US, the combined climate-change and health costs are $469 million for gasoline, $472–952 million for corn ethanol depending on biorefinery heat source (natural gas, corn stover, or coal) and technology, but only $123–208 million for cellulosic ethanol depending on feedstock (prairie biomass, Miscanthus, corn stover, or switchgrass).[87]
Efficiency of common crops
As ethanol yields improve or different feedstocks are introduced, ethanol production may become more economically feasible in the US. Currently, research on improving ethanol yields from each unit of corn is underway using biotechnology. Also, as long as oil prices remain high, the economical use of other feedstocks, such as cellulose, become viable. By-products such as straw or wood chips can be converted to ethanol. Fast growing species like switchgrass can be grown on land not suitable for other cash crops and yield high levels of ethanol per unit area.[62]
| Crop | Annual yield (Liters/hectare, US gal/acre) | Greenhouse-gas savings vs. petrol[a] |
Comments | |
|---|---|---|---|---|
| Miscanthus | 7300 L/ha, 780 g/acre |
37%–73% | Low-input perennial grass. Ethanol production depends on development of cellulosic technology. | |
| Switchgrass | 3100–7600 L/ha, 330–810 g/acre |
37%–73% | Low-input perennial grass. Ethanol production depends on development of cellulosic technology. Breeding efforts underway to increase yields. Higher biomass production possible with mixed species of perennial grasses. | |
| Poplar | 3700–6000 L/ha, 400–640 g/acre |
51%–100% | Fast-growing tree. Ethanol production depends on development of cellulosic technology. Completion of genomic sequencing project will aid breeding efforts to increase yields. | |
| Sugar cane | 6800–8000 L/ha,[59][88][89][90] 727–870 g/acre |
87%–96% | Long-season annual grass. Used as feedstock for most bioethanol produced in Brazil. Newer processing plants burn residues not used for ethanol to generate electricity. Grows only in tropical and subtropical climates. | |
| Sweet sorghum | 2500–7000 L/ha, 270–750 g/acre |
No data | Low-input annual grass. Ethanol production possible using existing technology. Grows in tropical and temperate climates, but highest ethanol yield estimates assume multiple crops per year (possible only in tropical climates). Does not store well.[91][92][93][94] | |
| Corn | 3100–4000 L/ha,[59][88][89][90] 330–424 g/acre |
10%–20% | High-input annual grass. Used as feedstock for most bioethanol produced in USA. Only kernels can be processed using available technology; development of commercial cellulosic technology would allow stover to be used and increase ethanol yield by 1,100 – 2,000 litres/ha. | |
| Source (except those indicated): Nature 444 (7 December 2006): 673–676. [a] – Savings of GHG emissions assuming no land use change (using existing crop lands). | ||||
Reduced petroleum imports and costs
One rationale given for extensive ethanol production in the U.S. is its benefit to energy security, by shifting the need for some foreign-produced oil to domestically produced energy sources.[95][96] Production of ethanol requires significant energy, but current U.S. production derives most of that energy from coal, natural gas and other sources, rather than oil.[97] Because 66% of oil consumed in the U.S. is imported, compared to a net surplus of coal and just 16% of natural gas (2006 figures),[98] the displacement of oil-based fuels to ethanol produces a net shift from foreign to domestic U.S. energy sources.
According to a 2008 analysis by Iowa State University, the growth in US ethanol production has caused retail gasoline prices to be US $0.29 to US $0.40 per gallon lower than would otherwise have been the case.[99]
Criticism
There are various social, economic, environmental and technical issues with biofuel production and use, which have been discussed in the popular media and scientific journals. These include: the effect of moderating oil prices, the "food vs fuel" debate, poverty reduction potential, carbon emissions levels, sustainable biofuel production, deforestation and soil erosion, loss of biodiversity, impact on water resources, as well as energy balance and efficiency[citation needed].
Motorsport
Leon Duray qualified third for the 1927 Indianapolis 500 auto race with an ethanol-fueled car.[100] The IndyCar Series adopted a 10% ethanol blend for the 2006 season, and a 98% blend in 2007.
In drag racing, there are Top Alcohol classes for dragsters and funny cars since the 1970s.
The American Le Mans Series sports car championship introduced E10 in the 2007 season to replace pure gasoline. In the 2008 season, E85 was allowed in the GT class and teams began switching to it.[101]
In 2011, the three national NASCAR stock car series mandated a switch from gasoline to E15, a blend of Sunoco GTX unleaded racing fuel and 15% ethanol.[102]
Ethanol fuel may also be utilized as a rocket fuel. As of 2010, small quantities of ethanol are used in lightweight rocket-racing aircraft.[103]
Replacement of kerosene
There is still extensive use of kerosene for lighting and cooking in less developed countries, and ethanol can have a role in reducing petroleum dependency in this use too. A non-profit named Project Gaia seeks to spread the use of ethanol stoves to replace wood, charcoal and kerosene.[104] There is also potential for bioethanol replacing some kerosene use in domestic lighting from feedstocks grown locally. A 50% ethanol water mixture has been tested in specially designed stoves and lanterns for rural areas.[citation needed]
Bibliography
- J. Goettemoeller, A. Goettemoeller (2007). Sustainable Ethanol: Biofuels, Biorefineries, Cellulosic Biomass, Flex-Fuel Vehicles, and Sustainable Farming for Energy Independence (Brief and comprehensive account of the history, evolution and future of ethanol). Prairie Oak Publishing,Maryville, Missouri. ISBN 978-0-9786293-0-4.
- Onuki, Shinnosuke; Koziel, Jacek A.; van Leeuwen, Johannes; Jenks, William S.; Grewell, David; Cai, Lingshuang (June 2008). "Ethanol production, purification, and analysis techniques: a review". 2008 ASABE Annual International Meeting. Providence, RI. Retrieved February 16, 2013.
- The Worldwatch Institute (2007). Biofuels for Transport: Global Potential and Implications for Energy and Agriculture (Global view, includes country study cases of Brazil, China, India and Tanzania). Earthscan Publications Ltd., London, U.K. ISBN 978-1-84407-422-8.
See also
- Alcohol fuel
- Biobutanol, a gasoline replacement.
- Bioconversion of biomass to mixed alcohol fuels
- Biomass
- Cellulosic ethanol
- Corn Ethanol
- Common ethanol fuel mixtures
- DMF (potential ethanol competitor biofuel)
- Dimethyl ether
- Energy crop
- Ethanol effect
- Ethanol from coal
- Flexible-fuel vehicle
- Gasoline gallon equivalent
- Indirect land use change impacts of biofuels
- Hydrogen fuel
- Liquid fuels
- Methanol fuel
- Low-carbon fuel standard
- P-series fuels
- Timeline of alcohol fuel
References
- ↑ 1.0 1.1 1.2 "Part One" (PDF). Retrieved 27 August 2011.
- ↑ "Towards Sustainable Production and Use of Resources: Assessing Biofuels". United Nations Environment Programme. 16 October 2009. Retrieved 24 October 2009.
- ↑ 3.0 3.1 3.2 3.3 3.4 Renewable Fuels Association (6 March 2012). "Acelerating Industry Innovation – 2012 Ethanol Industry Outlook". Renewable Fuels Association. Retrieved 18 March 2012. See pp. 3, 8, 10 22 and 23.
- ↑ "Gasoline Gallon Equivalent (GGE) Definition". energy.gov. Retrieved 12 October 2011.
- ↑ Worldwatch Institute and Center for American Progress (2006). American energy: The renewable path to energy security
- ↑ "Portaria Nº 143, de 27 de Junho de 2007" (in Portuguese). Ministério da Agricultura, Pecuária e Abastecimento. Retrieved 5 October 2008.
- ↑ "Anúario da Industria Automobilistica Brasileira 2011: Tabela 2.3 Produção por combustível – 1957/2010" (in Portuguese). ANFAVEA – Associação Nacional dos Fabricantes de Veículos Automotores (Brasil). Retrieved 22 January 2012. pp. 62–63.
- ↑ Renavam/Denatran (January 2012). "Licenciamento total de automóveis e comerciais leves por combustível" [Total automobiles and light-trucks registered by fuel] (in Portuguese). ANFAVEA. Retrieved 21 January 2012. Carta de ANFAVEA 308 pp. 4.
- ↑ Abraciclo (27 January 2010). "Motos flex foram as mais vendidas em 2009 na categoria 150cc" (in Portuguese). UNICA. Retrieved 10 February 2010.
- ↑ "Produção Motocicletas 2010" (in Portuguese). ABRACICLO. Retrieved 5 February 2011.
- ↑ "Produção Motocicletas 2011" [2011 Motorcycle Production] (in Portuguese). ABRACICLO. Retrieved 21 January 2012.
- ↑ "Deforestation diesel – the madness of biofuel" (PDF). Retrieved 27 August 2011.
- ↑ Youngquist, W. Geodestinies, National Book company, Portland, OR, 499p.
- ↑ "The dirty truth about biofuels". Oilcrash.com. 14 March 2005. Retrieved 27 August 2011.
- ↑ Kinver, Mark (18 September 2006). "Biofuels look to the next generation". BBC News. Retrieved 27 August 2011.
- ↑ O. R. Inderwildi, D. A. King (2009). "Quo Vadis Biofuels". Energy & Environmental Science 2 (4): 343. doi:10.1039/b822951c.
- ↑ 17.0 17.1 Biotechnology Industry Organization (2007). Industrial Biotechnology Is Revolutionizing the Production of Ethanol Transportation Fuel pp. 3–4.
- ↑ 18.0 18.1 International Energy Agency (2006). World Energy Outlook 2006 p. 8.
- ↑ "http://www.cato.org file v30n3-1" (PDF). Retrieved 11 October 2013.
- ↑ "meti.go.jp file g30819b40j" (PDF). Retrieved 27 August 2011.
- ↑ "(grainscouncil.com, Biofuels_study 268 kB pdf, footnote, p 6)". Web.archive.org. 18 July 2008. Archived from the original on 18 July 2008. Retrieved 27 August 2011.
- ↑ ethanolproducer.com, article 2077
- ↑ By: Martin LaMonica (12 June 2008). "Algae farm in Mexico to produce ethanol in '09". News.cnet.com. Retrieved 27 August 2011.
- ↑ "New Enzyme for More Efficient Corn Ethanol Production". Green Car Congress. 30 June 2005. Retrieved 14 January 2008.
- ↑ "Gasoline C made with Hydrous Ethanol in Brazil". Delphi South America Technical Center – Brazil. 30 July 2008.
- ↑ "Modern Corn Ethanol plant description" (PDF).
- ↑ 27.0 27.1 www.afdc.energy.gov Energy.gov site
- ↑ 28.0 28.1 www.eia.doe.gov Alternative Fuel Efficiencies in Miles per Gallon
- ↑ 29.0 29.1 "washington.edu, course, 22 October v2". Courses.washington.edu. Retrieved 27 August 2011.
- ↑ "Efficiency Improvements Associated with Ethanol-Fueled Spark-Ignition Engines". Swri.edu. 21 January 2011. Retrieved 27 August 2011.
- ↑ N. Stauffer (25 October 2006). "MIT's pint-sized car engine promises high efficiency, low cost". MIT. Retrieved 14 January 2008.
- ↑ Michael Millikin. "Squeezing More Out of Ethanol". HybridCars.com. Archived from the original on 28 August 2007. Retrieved 9 January 2013.
- ↑ Brinkman, N., Halsall, R., Jorgensen, S.W., & Kirwan, J.E., "The Development of Improved Fuel Specifications for Methanol (M85) and Ethanol (Ed85), SAE Technical Paper 940764
- ↑ W. Horn and F. Krupp. Earth: The Sequel: The Race to Reinvent Energy and Stop Global Warming. 2006, 85
- ↑ Mechanics see ethanol damaging small engines, msnbc.com, 8 January 2008
- ↑ "Microsoft Word - Direct_Injection_03=08=05_1.doc" (PDF). Retrieved 27 August 2011.
- ↑ "SAE Paper 2001-01-2901". Sae.org. 16 October 2000. Retrieved 27 August 2011.
- ↑ M. Brusstar, M. Bakenhus. "Economical, High-Efficiency Engine Technologies for Alcohol Fuels" (PDF). U.S. Environmental Protection Agency. Retrieved 14 January 2008.
- ↑ "Scania continues renewable fuel drive, New highly efficient diesel ethanol engine-- ready to cut fossil CO2 emissions by 90%" Scania PRESSInfo, 21 May 2007
- ↑ "England receives ethanol buses Brian Warshaw, Ethanol Producer, 21 March 2008
- ↑ Roman M. Balabin, et al. (2007). "Molar enthalpy of vaporization of ethanol–gasoline mixtures and their colloid state". Fuel 86 (3): 323. doi:10.1016/j.fuel.2006.08.008.
- ↑ "Sustainable biofuels: prospects and challenges" (PDF). The Royal Society. January 2008. Retrieved 27 September 2008. Policy document 01/08. See 4.3.1 Vapour pressure and bioethanol and Figure 4.3 for the relation between ethanol content and vapor pressure.
- ↑ Ethanol Promotion and Information Council (27 February 2007). "When is E85 not 85 percent ethanol? When it’s E70 with an E85 sticker on it". AutoblogGreen. Retrieved 24 August 2008.
- ↑ 44.0 44.1 "Ethanol fuel and cars". Interesting Energy Facts. Retrieved 23 September 2008.
- ↑ Vägverket (Swedish Road Administration) (30 May 2007). "Swedish comments on Euro 5/6 comitology version 4, 30 May 2007: Cold Temperature Tests For Flex Fuel Vehicles" (PDF). European Commission. Retrieved 23 September 2008.
- ↑ "Here comes the 'Flex' vehicles third generation" (PDF). Revista Brasileira de BioEnergia (in Portuguese, English) (Centro Nacional de Referência em Biomassa (Cenbio)). August 2008. Retrieved 23 September 2008. Ano 2, No. 3 (every article is presented in both English and Portuguese)
- ↑ Agência Estado (10 June 2008). "Bosch investe na segunda geração do motor flex" (in Portuguese, English). Gazeta do Povo. Retrieved 23 September 2008.
- ↑ Q. Rodas (March 2009). "Volkswagen Polo E-Flex" (in Portuguese). Editora Abril. Retrieved 12 March 2003.
- ↑ "Volks lança sistema que elimina tanquinho de gasolina para partida a frio" (in Portuguese). UNICA. 12 March 2009. Retrieved 12 March 2003.
- ↑ This is shown for 25 °C (77 °F) in a gasoline-ethanol-water phase diagram, Fig 13 of Päivi Aakko; Nils-Olof Nylund. "Technical View on Biofuels for Transportation – Focus on Ethanol End-Use Aspects" (PDF). Archived from the original on 3 December 2007. Retrieved 14 January 2008.
- ↑ as shown in Figure 1 of http://www.epa.gov/OMS/regs/fuels/rfg/waterphs.pdf
- ↑ Julieta Andrea Puerto Rico (8 May 2008). "Programa de Biocombustíveis no Brasil e na Colômbia: uma análise da implantação, resultados e perspectivas" (in Portuguese). Universidade de São Paulo. Retrieved 5 October 2008. PhD Dissertation Thesis, pp. 81–82
- ↑ "2011 Ethanol Industry Outlook: Building Bridges to a More Sustainable Future". Renewable Fuels Association. 2011. Retrieved 30 April 2011.See pages 2–3, 10–11, 19–20, and 26–27.
- ↑ Matthew L. Wald (13 October 2010). "A Bit More Ethanol in the Gas Tank". New York Times. Retrieved 14 October 2010.
- ↑ Fred Meier (13 October 2010). "EPA allows 15% ethanol in gasoline, but only for late-model cars". USA Today. Retrieved 14 October 2010.
- ↑ http://www.eia.doe.gov DOE FAQ
- ↑ "Ethanol in Petrol". Royal Automobile Association of South Australia. February 2004. Retrieved 29 April 2007.
- ↑ "EPA Info". US EPA. 7 March 2011. Retrieved 27 August 2011.
- ↑ 59.0 59.1 59.2 J. Goettemoeller, A. Goettemoeller (2007). Sustainable Ethanol: Biofuels, Biorefineries, Cellulosic Biomass, Flex-Fuel Vehicles, and Sustainable Farming for Energy Independence. Prairie Oak Publishing, Maryville, Missouri. p. 42. ISBN 978-0-9786293-0-4.
- ↑ "EPA Mileage". Fueleconomy.gov. Retrieved 27 August 2011.
- ↑ "Changes in Gasoline IV, sponsored by Renewable Fuels Foundation" (PDF). Retrieved 27 August 2011.
- ↑ 62.0 62.1 62.2 62.3 62.4 Green Dreams J.K. Bourne JR, R. Clark National Geographic Magazine October 2007 p. 41 Article
- ↑ "Home Mini-Refinery Makes Ethanol & Biodiesel Simultaneously". Gas2.0. 4 November 2008. Retrieved 4 November 2008.
- ↑ "Micro Fueler Is First Ethanol Kit for Brewing Backyard Biofuels on the Cheap". PopularMechanics. 8 May 2008. Retrieved 8 May 2008.
- ↑ F.O. Lichts. "Industry Statistics: 2010 World Fuel Ethanol Production". Renewable Fuels Association. Retrieved 30 April 2011.
- ↑ "2009 Global Ethanol Production (Million Gallons)". F.O. Licht, cited in Renewable Fuels Association, Ethanol Industry Overlook 2010, pp. 2 and 22. 2010. Retrieved 12 February 2011.
- ↑ F.O. Licht. "2007 and 2008 World Fuel Ethanol Production". Renewable Fuels Association. Archived from the original on 8 April 2008. Retrieved 17 April 2010.
- ↑ iea.org, biofuels2004.pdf
- ↑ Sanders, Robert (26 January 2006).Ethanol can replace gasoline with significant energy savings, comparable impact on greenhouse gases. University of California Berkley Energy Resources Group, Dan Kammen and Alex Farrell; Michael O'Hare, Goldman School of Public Policy. Also published 27 January 2006 VOL 311 Science, www.sciencemag.org .Retrieved 22 August 2011.
- ↑ "oregon.gov, biomass forum". Oregon.gov. 27 March 2009. Retrieved 27 August 2011.
- ↑ M. Wang, C. Saricks, D. Santini. "Effects of Fuel Ethanol Use on Fuel-Cycle Energy and Greenhouse Gas Emissions" (PDF). Argonne National Laboratory. Retrieved 7 July 2009.
- ↑ M. Wang. "Energy and Greenhouse Gas Emissions Effects of Fuel Ethanol" (PDF). Retrieved 7 July 2009.
- ↑ Davidson, Keay (18 April 2007). "Study warns of health risk from ethanol". San Francisco Chronicle. Retrieved 7 July 2009.
- ↑ "Clearing the air on ethanol". Environmental Science & Technology. 18 April 2007. Retrieved 14 January 2008.
- ↑ M. Z. Jacobson (14 March 2007). "Effects of Ethanol (E85) vs. Gasoline Vehicles on Cancer and Mortality in the United States". ACS Publications. Retrieved 14 January 2008.
- ↑ itsallaboutyou (25 July 2011). "CBS News". CBS News. Retrieved 27 August 2011.
- ↑ Bioethanol Production and Use Creating Markets for Renewable Energy Technologies EU, RES Technology Marketing Campaign, European Biomass Industry Association EUBIA 2007
- ↑ 78.0 78.1 New York Times, Biofuels Deemed a Greenhouse Threat Biofuels Deemed a Greenhouse Threat by Rosenthal, 8 February 2008.
- ↑ Sciencexpress Report Land Clearing and the Biofuel Carbon Debt Fargione, Hill, Tilman, Polasky, and Hawthorne. 7 February 2008.
- ↑ 80.0 80.1 D. Morrison (18 September 2006). "Ethanol fuel presents a corn-undrum". University of Minnesota. Retrieved 14 January 2008.
- ↑ "Lula calls for ethanol investment". BBC. 4 June 2007. Retrieved 14 January 2008.
- ↑ "Brazil's ethanol push could eat away at Amazon". Associated Press. 7 March 2007. Retrieved 14 January 2008.
- ↑ Kononova, M. M. Soil Organic Matter, Its Nature, Its role in Soil Formation and in Soil Fertility, 1961
- ↑ D. Russi (7 March 2007). "Biofuels: An advisable strategy?". Archived from the original on 29 March 2008.
- ↑ Greater Transportation Energy and GHG Offsets from Bioelectricity Than Ethanol Campbell, et al. Science 22 May 2009: 1055–1057. DOI:10.1126/science.1168885
- ↑ Block, Ben, "Study: biofuels more efficient as electricity source.(EYE ON EARTH)(Brief article)" World Watch 22.
- ↑ Hill, Jason, Stephen Polasky, Erik Nelson, David Tilman, Hong Huo, Lindsay Ludwig, James Neumann, Haochi Zheng, and Diego Bonta. "Climate change and health costs of air emissions from biofuels and gasoline.(SUSTAINABILITY SCIENCE)(Author abstract)." Proceedings of the National Academy of Sciences of the United States 106.6 (10 February 2009): 2077(6). Expanded Academic ASAP. Gale. BENTLEY UPPER SCHOOL LIBRARY (BAISL). 6 October 2009
- ↑ 88.0 88.1 D. Budny, P. Sotero (April 2007). "Brazil Institute Special Report: The Global Dynamics of Biofuels" (PDF). Brazil Institute of the Woodrow Wilson Center (updated to Jan 2011). Retrieved 3 May 2008.
- ↑ 89.0 89.1 J. Duailibi (27 April 2008). "Ele é o falso vilão" (in Portuguese). Veja Magazine. Retrieved 3 May 2008.
- ↑ 90.0 90.1 M. H. Tachinardi (13 June 2008). "Por que a cana é melhor que o milho" (in Portuguese). Época Magazine. Retrieved 6 August 2008. Print edition pp. 73
- ↑ Belum V S Reddy; A Ashok Kumar and S Ramesh. "Sweet sorghum: A Water Saving BioEnergy Crop" (PDF). International Crops Research Institute for the SemiArid Tropics. Retrieved 14 January 2008.
- ↑ "RP INVESTOR TO PUT UP PIONEERING SWEET SORGHUM ETHANOL PLANT". Manila Bulletin. 25 October 2006. Retrieved 14 January 2008.
- ↑ G. C. Rains, J. S. Cundiff, and G. E. Welbaum (12 September 1997). "Sweet Sorghum for a Piedmont Ethanol Industry". Retrieved 14 January 2008.
- ↑ "ICRISAT develops sweet sorghum for ethanol production". 12 August 2004. Archived from the original on 15 December 2007. Retrieved 14 January 2008.
- ↑ "Energy Security". Ethanol.org. Retrieved 27 August 2011.
- ↑ M. Turon (25 November 1998). Ethanol as Fuel: An Environmental and Economic Analysis. U.C. Berkeley, Chemical Engineering.
- ↑ "Ethanol Can Contribute to Energy and Environmental Goals". Ethanol.org. Retrieved 27 August 2011.
- ↑ "Energy INFOcard". Eia.doe.gov. Retrieved 27 August 2011.
- ↑ "Ethanol Lowers Gas Prices 29–40 Cents Per Gallon". Renewableenergyworld.com. Retrieved 27 August 2011.
- ↑ IRL Switch to Ethanol May Be Precursor for Passenger Cars - Motor Magazine, August 2006
- ↑ ALMS Corvettes going green with E85 fuel in 2008 - USA Today, 15 January 2008
- ↑ NASCAR to shift to E15 fuel in 2011 - Fox Sports, 16 October 2010
- ↑ Rocket Racing League Unveils New Flying Hot Rod, by Denise Chow, Space.com, 2010-04-26. Retrieved 27 April 2010.
- ↑ "Welcome to Project Gaia". Project Gaia. Retrieved 6 May 2009.
External links
- Towards Sustainable Production and Use of Resources: Assessing Biofuels, United Nations Environment Programme, October 2009
- Ethanol blended petrol usage in Brazil
- World Bank, Biofuels: The Promise and the Risks. World Development Report 2008: Agriculture for Development
- Biofuels: Ethanol on the Open Directory Project
- Ethanol Better Than Gasoline, If Done Right
| |||||||||||||||||||
| ||||||||||||||||


