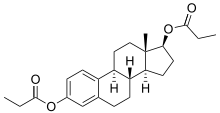
Estradiol dipropionate
 | |
|---|---|
| Systematic (IUPAC) name | |
| (17β)-estra-1,3,5(10)-triene-3,17-diyl dipropanoate | |
| Clinical data | |
| Legal status | ℞ Prescription only |
| Routes | IM |
| Identifiers | |
| CAS number | 113-38-2 |
| ATC code | None |
| PubChem | CID 8225 |
| ChemSpider | 7932 |
| Synonyms | 17β-Estradiol-3,17-dipropionate |
| Chemical data | |
| Formula | C24H32O4 |
| Mol. mass | 384.509 g/mol |
| SMILES
| |
| |
Estradiol dipropionate (BAN, JAN; brand names Agofollin, Diovocyclin, Progynon-DP, others) is a synthetic ester, specifically the 3,17-dipropanoyl ester, of the natural estrogen, estradiol.[1][2] Alongside estradiol benzoate, estradiol dipropionate was one of the first estradiol esters to be developed, having been patented in 1937,[3] and has been marketed since at least the 1940s.[4]
See also
References
- ↑ A. D. Roberts (1991). Dictionary of Steroids: Chemical Data, Structures, and Bibliographies. CRC Press. p. 415. ISBN 978-0-412-27060-4. Retrieved 20 May 2012.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 406. ISBN 978-3-88763-075-1. Retrieved 20 May 2012.
- ↑ States2233025 United States 2233025, Karl Miescher, Riehen, & Caesar Scholz, "Estradiol-17-monoesters", published 1941-02-25, assigned to Ciba Pharmaceutical Products, Inc.
- ↑ Reece RP, Leathem, JH (June 1945). "Growth of mammary glands of hypophysectomized rats following estrogen and lactogen administration". Experimental Biology and Medicine 59 (2): 122–124. doi:10.3181/00379727-59-15001.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||