Eseroline
From Wikipedia, the free encyclopedia
 | |
|---|---|
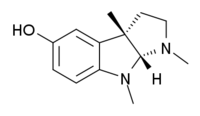
| Systematic (IUPAC) name | |
| (3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-ol | |
| Clinical data | |
| Legal status | ? |
| Identifiers | |
| CAS number | 469-22-7 |
| ATC code | None |
| PubChem | CID 119198 |
| ChemSpider | 106485 |
| Synonyms | Eseroline |
| Chemical data | |
| Formula | C13H18N2O |
| Mol. mass | 218.295 g/mol |
| SMILES
| |
| |
| | |
Eseroline is a drug which acts as an opioid agonist.[1] It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible,[2][3] and it produces fairly potent analgesic effects mediated through the μ-opioid receptor.[4] This mixture of activities gives eseroline an unusual pharmacological profile,[5][6] although its uses are limited by side effects such as respiratory depression[7] and neurotoxicity.[8]
References
- ↑ Fürst, S; Friedmann, T; Bartolini, A; Bartolini, R; Aiello-Malmberg, P; Galli, A; Somogyi, GT; Knoll, J (1982). "Direct evidence that eseroline possesses morphine-like effects". European Journal of Pharmacology 83 (3–4): 233–41. doi:10.1016/0014-2999(82)90256-4. PMID 6293841.
- ↑ Jhamandas, K; Elliott, J; Sutak, M (1981). "Opiatelike actions of eseroline, an eserine derivative". Canadian journal of physiology and pharmacology 59 (3): 307–10. PMID 7194726.
- ↑ Galli, A; Renzi, G; Grazzini, E; Bartolini, R; Aiello-Malmberg, P; Bartolini, A (1982). "Reversible inhibition of acetylcholinesterase by eseroline, an opioid agonist structurally related to physostigmine (eserine) and morphine". Biochemical pharmacology 31 (7): 1233–8. doi:10.1016/0006-2952(82)90009-0. PMID 7092918.
- ↑ Agresti, A; Buffoni, F; Kaufman, JJ; Petrongolo, C (1980). "Structure--activity relationships of eseroline and morphine: ab initio quantum-chemical study of the electrostatic potential and of the interaction energy with water". Molecular Pharmacology 18 (3): 461–7. PMID 7464812.
- ↑ Galli, A; Ranaudo, E; Giannini, L; Costagli, C (1996). "Reversible inhibition of cholinesterases by opioids: possible pharmacological consequences". The Journal of pharmacy and pharmacology 48 (11): 1164–8. doi:10.1111/j.2042-7158.1996.tb03914.x. PMID 8961166.
- ↑ Liu, WF (1991). "Effect of eseroline on schedule-controlled behavior in the rat". Pharmacology, Biochemistry, and Behavior 38 (4): 747–51. doi:10.1016/0091-3057(91)90236-U. PMID 1871191.
- ↑ Berkenbosch, A; Rupreht, J; Degoede, J; Olievier, CN; Wolsink, JG (1993). "Effects of eseroline on the ventilatory response to CO2". European Journal of Pharmacology 232 (1): 21–8. doi:10.1016/0014-2999(93)90723-U. PMID 8458393.
- ↑ Somani, SM; Kutty, RK; Krishna, G (1990). "Eseroline, a metabolite of physostigmine, induces neuronal cell death". Toxicology and applied pharmacology 106 (1): 28–37. doi:10.1016/0041-008X(90)90102-Z. PMID 2251681.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.