Erythritol
| Erythritol | |
|---|---|
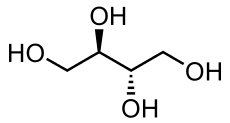 | |
| IUPAC name (2R,3S)-butane-1,2,3,4-tetraol | |
| Identifiers | |
| CAS number | 10030-58-7 |
| PubChem | 222285 |
| ChemSpider | 192963 |
| UNII | RA96B954X6 |
| DrugBank | DB04481 |
| KEGG | D08915 |
| ChEBI | CHEBI:17113 |
| ChEMBL | CHEMBL349605 |
| Jmol-3D images | {{#if:OC[C@@H](O)[C@@H](O)COC([C@H]([C@H](CO)O)O)O|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C4H10O4 |
| Molar mass | 122.12 g mol−1 |
| Density | 1.45 g/cm³ |
| Melting point | 121 °C; 250 °F; 394 K |
| Boiling point | 329 to 331 °C; 624 to 628 °F; 602 to 604 K |
| Hazards | |
| NFPA 704 |
 1
1
0
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Erythritol ((2R,3S)-butane-1,2,3,4-tetraol) is a sugar alcohol (or polyol) that has been approved for use as a food additive in the United States[1] and throughout much of the world. It was discovered in 1848 by British chemist John Stenhouse.[2] It occurs naturally in some fruits and fermented foods.[3] At the industrial level, it is produced from glucose by fermentation with a yeast, Moniliella pollinis.[1] It is 60–70% as sweet as table sugar yet it is almost noncaloric, does not affect blood sugar, does not cause tooth decay, and is partially absorbed by the body, excreted in urine and feces. It is less likely to cause gastric side effects than other sugar alcohols because of its unique digestion pathway. Under U.S. Food and Drug Administration (FDA) labeling requirements, it has a caloric value of 0.2 kilocalories per gram (95% less than sugar and other carbohydrates), though nutritional labeling varies from country to country. Some countries, such as Japan and the United States, label it as zero-calorie, while European Union regulations currently label it and all other sugar alcohols at 0.24 kcal/g.
Erythritol and human digestion
In the body, most erythritol is absorbed into the bloodstream in the small intestine, and then for the most part excreted unchanged in the urine. About 10% enters the colon.[4] Because 90% of erythritol is absorbed before it enters the large intestine, it does not normally cause laxative effects, as are often experienced after consumption of other sugar alcohols (such as xylitol and maltitol),[5] although extremely large doses can cause nausea and borborygmi.[6]
Side effects
Doses over 50 grams (1.8 oz) can cause a significant increase in nausea and borborygmi (stomach rumbling),[6] and (rarely) erythritol can cause allergic urticaria (hives).[7]
In general, erythritol is free of side effects in regular use. When compared with other sugar alcohols, it is also much more difficult for intestinal bacteria to digest, so is less likely to cause gas or bloating than other polyols,[8] such as maltitol, sorbitol, or lactitol.
Physical properties
Heat of solution
Erythritol has a strong cooling effect (endothermic, or negative heat of solution)[9] when it dissolves in water, which is often combined with the cooling effect of mint flavors. The cooling effect is present only when erythritol is not already dissolved in water, a situation that might be experienced in an erythritol-sweetened frosting, chocolate bar, chewing gum, or hard candy. The cooling effect of erythritol is very similar to that of xylitol and among the strongest cooling effects of all sugar alcohols.[10]
Blending for sugar-like properties
Erythritol is commonly used as a medium in which to deliver high-intensity sweeteners, especially stevia derivatives, serving the dual function of providing both bulk and a flavor similar to that of table sugar. Diet beverages made with this blend, thus, contain erythritol in addition to the main sweetener. Beyond high-intensity sweeteners, erythritol is often paired with other bulky ingredients that exhibit sugar-like characteristics to better mimic the texture and mouthfeel of sucrose. The cooling effect of erythritol is rarely desired, hence other ingredients are chosen to dilute or negate that effect. Erythritol also has a propensity to crystallize and is not as soluble as sucrose, so ingredients may also be chosen to help negate this disadvantage. Furthermore, erythritol is not hygroscopic, meaning it does not attract moisture, which can lead to the drying out of products, in particular baked goods, if another hygroscopic ingredient is not used in the formulation.
Inulin is often combined with erythritol because of inulin's offering a complementary negative heat of solution (exothermic, or warming effect when dissolved, which helps cancel erythritol's cooling effect) and noncrystallizing properties. However, inulin has a propensity to cause gas and bloating in those having consumed it in moderate to large quantities, in particular in individuals unaccustomed to it. Other sugar alcohols are sometimes used with erythritol, in particular isomalt, because of its minimally positive heat of solution, and glycerin, which has a negative heat of solution, moderate hygroscopicity, and noncrystallizing liquid form.
Erythritol and bacteria
Erythritol has been certified as tooth-friendly.[11] The sugar alcohol cannot be metabolized by oral bacteria, so does not contribute to tooth decay. Erythritol exhibits some, but not all, of xylitol's tendency to "starve" harmful bacteria.[citation needed] Unlike xylitol, erythritol is actually absorbed into the bloodstream after consumption but before excretion. However, it is not clear at present if the effect of starving harmful bacteria occurs systemically.
See also
- Threitol, the diastereomer of erythritol
- Stevia
- Erythritol tetranitrate
References
- ↑ 1.0 1.1 FDA/CFSAN: Agency Response Letter: GRAS Notice No. GRN 000076
- ↑ The discovery of erythritol, which Stenhouse called "erythroglucin", was announced in: Stenhouse, John (January 1, 1848). "Examination of the proximate principles of some of the lichens". Philosophical Transactions of the Royal Society of London 138: 63–89; see especially p. 76.
- ↑ Shindou, T., Sasaki, Y., Miki, H., Eguchi, T., Hagiwara, K., Ichikawa, T. (1988). "Determination of erythritol in fermented foods by high performance liquid chromatography". Shokuhin Eiseigaku Zasshi 29 (6): 419–422.
- ↑ Arrigoni, E.; Brouns, F.; Amadò, R. (Nov 2005). "Human gut microbiota does not ferment erythritol.". Br J Nutr 94 (5): 643–6. PMID 16277764.
- ↑ Munro IC, Berndt WO, Borzelleca JF, et al. (December 1998). "Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data". Food Chem. Toxicol. 36 (12): 1139–74. doi:10.1016/S0278-6915(98)00091-X. PMID 9862657.
- ↑ 6.0 6.1 Storey, D.; Lee, A.; Bornet, F.; Brouns, F. (Mar 2007). "Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid.". Eur J Clin Nutr 61 (3): 349–54. doi:10.1038/sj.ejcn.1602532. PMID 16988647.
- ↑ Hino, H.; Kasai, S.; Hattori, N.; Kenjo, K. (Mar 2000). "A case of allergic urticaria caused by erythritol.". J Dermatol 27 (3): 163–5. PMID 10774141.
- ↑ Arrigoni E, Brouns F, Amadò R (November 2005). "Human gut microbiota does not ferment erythritol". Br. J. Nutr. 94 (5): 643–6. PMID 16277764.
- ↑ Wohlfarth, Christian (2006). CRC handbook of enthalpy data of polymer-solvent systems. CRC/Taylor & Francis. pp. 3–. ISBN 978-0-8493-9361-7.
- ↑ Jasra,R.V.; Ahluwalia, J.C. 1982. Enthalpies of Solution, Partial Molal Heat Capacities and Apparent Molal Volumes of Sugars and Polyols in Water. Journal of Solution Chemistry, 11 ( 5): 325-338. ISSN 1572-8927
- ↑ Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T (1992). "Noncariogenicity of erythritol as a substrate". Caries Res. 26 (5): 358–62. PMID 1468100.
| ||||||||||||||||||||||||||||||||