Equine protozoal myeloencephalitis
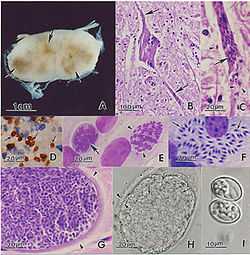
Equine protozoal myeloencephalitis, or EPM, is a disease caused by a protozoal infection by S. neurona that affects the central nervous system of horses.
History
EPM was first discovered in the 1960s by Dr. Jim Rooney. The disease is considered rare, though recently, an increasing number of cases have been reported. Research at the University of Kentucky identified the opossum as the definitive host of the disease. The term EPM refers to the clinical neurologic symptoms caused by the parasite, not infection itself. The majority of horses infected with S. neurona do not exhibit neurologic symptoms consistent with EPM. There are six subspecies of S. neurona which can be identified by surface antigens (SAG). Equine EPM is caused by the parasites that exhibit SAG1, SAG5, and SAG6. SAG1 and SAG5 are responsible for the majority of EPM cases in horses. Horses produce antibodies to these surface antigens. Serum antibody testing is available that measure levels of these antibodies in the blood of horses which is helpful in diagnosing EPM in an ataxic horse. Serial blood levels are helpful in guiding treatment. In experimentally infected horses it takes 17 days from infection to positive antibody tests. 80% of horses with EPM have positive antibody tests. A negative antibody test in the presence of EPM results if testing is done before 17 days or if the horse has been treated with antiprotozoal drugs which delays antibody production.
Causes

EPM is caused by the parasite Sarcocystis neurona. The life cycle of S. neurona is well described. In order to complete its life cycle this parasite needs two hosts, a definitive and an intermediate. In the laboratory, raccoons, cats, armadillos, skunks, and sea otters have been shown to be intermediate hosts. The opossum is the definitive host of the disease. Horses most commonly contract EPM from grazing or watering in areas where an opossum has recently defecated. However, horses cannot pass the disease among themselves, that is, one horse cannot contract the disease from another infected horse. The horse is a dead-end, or aberrant, host of the parasite.[1]
Symptoms
The neurologic signs that EPM causes are most commonly asymmetric incoordination (ataxia), weakness and spasticity, although they may mimic almost any neurologic disorder. Clinical signs among horses with EPM include a wide array of symptoms that may result from primary or secondary problems. Some of the signs are difficult to distinguish from other problems, such as lameness, which can be attributed to many different causes. Apparent lameness, particularly atypical lameness or slight gait asymmetry of the rear limbs are commonly caused by EPM. Focal muscle atrophy, or even generalized muscle atrophy or loss of condition may result. Secondary signs also occur with neurologic disease. Airway abnormalities, such as laryngeal hemiplegia (paralyzed flaps), dorsal displacement of the soft palate (snoring), or airway noise of undetermined origin may result from protozoa infecting the nerves which innervate the throat, although this is uncommon.
In experimentally infected horses, very early and in some cases transient signs included dropping feed, decreased tongue tone, facial paresis, mentation change, generalized weakness, and lameness.
It is thought that Sarcocystis neurona does not need to enter the CNS to cause disease, in some cases S. neurona has been found in the CNS but usually not. In cases where S. neurona is found in the CNS, leukocytes (white blood cells) probably play a roll in the parasite's penetration of the blood brain barrier.
Treatment and prevention
EPM is treatable, but irreversible damage to the nervous system is possible. It is important to identify the disease as early as possible and begin treatment with antiprotozoal drugs. There are currently two FDA approved treatments available in the US: Marquis (ponazuril), and Protazil (diclazuril). These drugs minimize the infection but do not kill the parasite. There is no longer an FDA approved sulfadiazine/pyrimethamine combination on the market. However, this combination can be prepared by compounding pharmacies and made available by prescription from a licensed veterinarian. The use of anti-inflammatory agents such as Banamine, corticosteroids, or phenylbutazone are often used to help reduce inflammation and limit further damage to the CNS. Antioxidants, such as vitamin E may help promote the restoration of nervous tissue. The response to treatment is often variable and may be expensive. Recently antiprotozoal treatment that kill the parasite and clear the infection have shown great promise. The inflammatory component is thought responsible for the symptoms of EPM and anti inflammatory drugs that target the IL-6 pathway have been particularly effective at reversing symptoms.
Control of this disease includes proper storage of hay and feed, the control of opossums on the property, and prompt disposal of animal carcasses. No vaccine is available.
References
http://www.aaep.org/health_articles_view.php?id=248 http://www.thehorse.com/free-reports/29984/equine-protozoal-myeloencephalitis-epm
Furr M, McKenzie H, Saville WJ, Dubey JP, Reed SM, Davis W. J Parasitol. 2006 Jun;92(3):637-43
Decoquinate Combined with Levamisole Reduce the Clinical Signs and Serum SAG 1, 5, 6 Antibodies in Horses with Suspected Equine Protozoal Myeloencephalitis,Siobhan P. Ellison, DVM, PhD, David S. Lindsay, PhD, Intern J Appl Res Vet Med • Vol. 10, No. 1, 2012
Dubey JP, Davis SW, Speer CA. Sarcocystis neurona N SP (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis.J. Parasitol 77:212-218, 1991.
Cutler T, MacKay RJ, Ginn PE, et al. Are Sarcocystis neurona and Sarcocystis falcatula synonymous? A horse infection challenge. J. Parasitol85:301-305, 1999.
Fenger CK, Granstrom DE, Gajadhar A, et al. Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp. sporocysts from the opossum (Didelphis virginiana). Vet Parasitol 68:199-213, 1997.
MacKay RJ. Serum antibodies to Sarcocystis neurona-half the horses in the United States have them! JAVMA 210:482-483, 1997.
Granstrom DE. Diagnosis of equine protozoal myeloencephalitis: Western blot analysis. Proc Am Coll Vet Intern Med Forum 587-590, 1993.
Granstrom DE, MacPherson JM, Gajadhar AA, et al. Differentiation of Sarcocystis neurona from eight related coccidia by random amplified polymorphic NA assay. J Molec Cellular Probes 8:353-356, 1994.
Hamir AN, Moser G, Galligan DT, et al. Immunohistochemical study to demonstrate Sarcocystis neurona in equine protozoal myeloencephalitis. J Vet Diagn Invest 5:418-422, 1993.
Furr M, MacKay R, Granstrom D, et al. J Vet Intern Med 16: 618-621, 2002.
Ellison SP, Omara-Opyeme AL, Yowell C, Dame J. Molecular characterization of a major 29 kDa surface antigen of Sarcocystis neurona. J Parasit 32:217-225, 2002.
Andrews FM. A review: Determining the sensitivity and specificity of western blot tests for diagnosis of Equine protozoal myeloencephalitis.Equine Med Rev
2003.11. Ellison SP. Development of a recombinant protein for the identification of S. neurona infections in horses. [PhD dissertation]. University of Florida, Gainesville, Florida, 2001.
Experimental induction of equine protozoan my-eloencephalitis (EPM) in the horse: effect of Sarco-cystis neurona sporocyst inoculation dose on thedevelopment of clinical neurologic disease. Sofaly Vol. 10, No. 1, 2012 • Intern J Appl Res Vet Med.CD, Reed SM, Gordon JC, Dubey JP, OgleebeeMJ, Njoku CJ, Grover DL, Saville WJ. 6, 2002, JParasitol, Vol. 88, pp. 1164-1170.
Levamisole, the story and the lessons. Amery, W K Pand Bruynseels, J M. 3, 1992, Inr. J Immunophar-mac, Vol. 14, pp. 481-86
Immunomodulation effects of various anti-parasitics:a review. Sajid, M S. 2006, Parasitol, Vol. 132, pp.301-13
Lindsay, David A. David Lindsay Decoquinate,4-hydroxyquinalones and hydroxyquinalones andnapthoquinones for the prevention and treatmentof equine protozoal myeloencephalitis caused bySarcocystis neurona. 2001.
Molecular characterisation of a major 29 kDa surfaceantigen of Sarcocystis neurona. Ellison SP, Omara-Opyene AL, Yowell CA, Marsh AE, Dame JB. 2,2002, Int J Parasitol., Vol. 32, pp. 217-25.
SnSAG5 is an alternative surface antigen of Sarco-cystis neurona strains that is. Crowdus CA, MarshAE, Saville WJ, Lindsay DS, Dubey JP, GranstromDE, Howe DK. 1-2, 2008, Vet Parasitol., Vol. 158,pp. 36-43
Ellison, Siobhan P. sarcocystis. Pathogenes Inc.[Online] Pathogenes Inc, 2003
Limited genetic diversity among Sarcocystis neu-rona strains infecting southern sea otters precludesdistinction between marine and terrestrial isolates.Wendte JM, Miller MA, Nandra AK, Peat SM,Crosbie PR, Conrad PA, Grigg ME. 1-2, Apr 2010,Vet Parasitol, Vol. 169, pp. 37-44
Development of an ELISA to detect antibodies torSAG 1 in the horse. S P Ellison, T Kennedy, KK Brown. 4, 2003, J App Res Vet Med, Vol. 1, pp.318-327
Experimental infection of horses with S. neurona merozoites as a model for Equine Protozoal My-eloencephalitis. Ellison, S. P., Greiner, E., Brown,K K., Kennedy, T. 2, 2004, J App Res Vet Med, Vol.2, pp. 79-89.
Characterization of a Sarcocystis neurona isolatefrom a Missouri horse with equine protozoalmyeloencephalitis. Marsh AE, Johnson PJ, Ramos-Vara J, Johnson GC. 2-4, Feb 2001, Vet Parasitol,Vol. 95, pp. 143-54.
Cytokine Gene Expression in Response to SnSAG1in Horses with Equine Protozoal Myeloencepha-litis. Spencer JA, Deinnocentes P, Moyana EM,Guarino AJ, Ellison SP, Bird RC, and BlagburnBL. 2004, J Parasitol.
Immune response to Sarcocystis neurona infectionin naturally infected horses with equine protozoalmyeloencephalitis. Yang J., Ellison S., GogalR., Norton H., Lindsay D S., Andrews R., WardR., Ward D., Witonsky. 3-4, 2006, Vol. 138, pp.200-10.
In vitro suppressed immune response in horsesexperimentally infected with Sarcocystis neurona.Witonsky S., Ellison S., Yang J., Gogal R., NortonH., Yasuhiro S., Sriranganathan N., Andrews F.,Ward D., Lindsay DS. 1, 2008, Vol. 12, p. 1.
Sarcocystis neurona n. sp. (Protozoa: Apicom-plexa), the etiologic agent of equine protozoalmyeloencephalitis. Dubey JP, Davis SW, SpeerCA, Bowman DD, de Lahunta A, Granstrom DE,Topper MJ, Hamir AN, Cummings JF, Suter MM.2, 1991, J Parasitol, Vol. 77, pp. 212-8.
Antibody index and specific antibody quotient inhorses after intragastric administration of Sarcocys-tis neurona sporocysts. Heskett KA, Mackay RJ. 3,Mar 2008, Am J Vet, Vol. 69, pp. 403-9.
An equine protozoal myeloencephalitis challengemodel testing a second transport after inoculationwith Sarcocystis neurona sporocysts. Saville WJ,Sofaly CD, Reed SM, Dubey JP, Oglesbee MJ,Lacombe VA, Keene RO, Gugisberg KM, SwensenSW, Shipley RD, Chiang YW, Chu HJ, Ng T. 6,2006, J Parasitol, Vol. 90, pp. 1406-10.
An equine protozoal myeloencephalitis challengemodel testing a second transport after inoculationwith Sarcocystis neurona sporocysts. Saville WJ,Sofaly CD, Reed SM, Dubey JP, Oglesbee MJ,Lacombe VA, Keene RO, Gugisberg KM, SwensenSW, Shipley RD, Chiang YW, Chu HJ, Ng T. 6,2002, J Parasitol., Vol. 88, pp. 1164-70.