Episterol
From Wikipedia, the free encyclopedia
| Episterol | ||
|---|---|---|
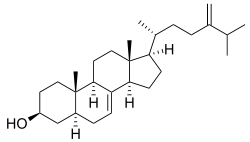 | ||
| IUPAC name (3S,5S,10S,13R,14R,17R)-10, 13-dimethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-2,3,4,5,6,9,11, 12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | ||
| Identifiers | ||
| CAS number | 57-87-4 | |
| PubChem | 47205103 | |
| MeSH | Episterol | |
| Jmol-3D images | {{#if:CC(C)C(=C)CC[C@@H](C)[C@H]1CC[C@H]2C3=CC[C@H]4C[C@@H](O)CC[C@]4(C)C3CC[C@]12C|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C28H46O | |
| Molar mass | 398.66 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Episterol is a sterol involved in the biosynthesis of steroids. Episterol is converted from 24-methylenelophenol. Episterol is converted to 5-dehydroepisterol by the enzyme lathosterol oxidase. Episterol is also known to be a precursor to ergosterol.
External links
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.