Epimer
In chemistry, epimers are stereoisomers that differ in configuration of only one stereogenic center other than last asymmetric carbon atom and anomeric carbon atom. All other stereocenters in the molecules, if any, are the same in each.
In chemical nomenclature, one of the epimeric pairs is given the prefix epi- for example in quinine and epi-quinine. When the pairs are enantiomers, the prefix becomes ent-.
Examples
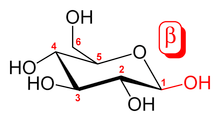
The sugars glucose and galactose are epimers. In glucose, the -OH group on the first carbon is in the direction opposite the methylene group on carbon C-4 (in the axial position). In galactose, the -OH group is oriented in the same direction as the methylene group (in the equatorial position).[1] These two molecules are both epimers and anomers (as indicated by the α-glucose and β designation).
 |  |
|
|
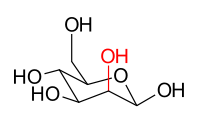
The stereoisomers β-D-glucopyranose and β-D-mannopyranose are epimers because they differ only in the stereochemistry at the C-2 position. The hydroxyl group in β-D-glucopyranose is equatorial (in the "plane" of the ring) while in β-D-mannopyranose the C-2 hydroxyl group is axial (up from the "plane" of the ring). These two molecules are epimers but, because not mirror images of each other, are also not enantiomers (enantiomers have the same name but differ in D and L classification). They are also not sugar anomers, since the wrong carbon is involved in the stereochemistry.
 |  |
| |
|
Doxorubicin and epirubicin are two closely related drugs and epimers.
 |
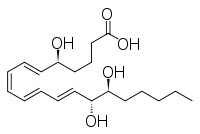
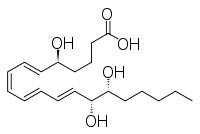
Other closely related compounds are epi-inositol and inositol and lipoxin and epilipoxin.
 |  |  |  |
Epimerisation
Epimerisation is a chemical process where an epimer is transformed into its chiral counterpart. It can happen in condensed tannins depolymerisation reactions. Epimerisation can be spontaneous (generally a slow process), or catalyzed by enzymes, e.g. the epimerization between the sugars N-acetylglucosamine and N-acetylmannosamine, which is catalyzed by Renin-Binding Protein.