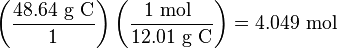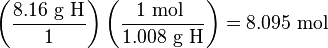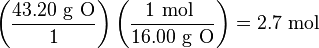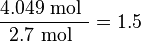Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.[1] A simple example of this concept is that the empirical formula of hydrogen peroxide, or H2O2, would simply be HO.
An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic compounds, such as CaCl2, and for macromolecules, such as SiO2.
In contrast, the molecular formula identifies the number of each type of atom in a molecule, and the structural formula also shows the structure of the molecule.
For example, glucose (C
6H
12O
6), ribose (C
5H
10O
5), acetic acid (C
2H
4O
2), and formaldehyde (CH
2O) all have different molecular formulas but the same empirical formula: CH
2O. This is the actual molecular formula for formaldehyde, but acetic acid has double the number of atoms, ribose has five times the number of atoms, and glucose has six times the number of atoms.
For example, the chemical compound n-hexane has the structural formula CH
3CH
2CH
2CH
2CH
2CH
3, which shows that it has 6 carbon atoms arranged in a chain, and 14 hydrogen atoms. Hexane's molecular formula is C
6H
14, and its empirical formula is C
3H
7, showing a C:H ratio of 3:7. Different compounds can have the same empirical formula.
Calculating Empirical Formulas
Suppose you are given a compound such as methyl acetate, a solvent commonly used in paints, inks, and adhesives. When methyl acetate was chemically analyzed, it was discovered to have 48.64% carbon (C), 8.16% hydrogen (H), and 43.20% oxygen (O). For the purposes of determining empirical formulas, we assume that we have 100 g of the compound. If this is the case, the percentages will be equal to the mass of each element in grams.
Step 1: Change each percentage to an expression of the mass of each element in grams. That is, 48.64% C becomes 48.64 g C, 8.16% H becomes 8.16 g H, and 43.20% O becomes 43.20 g O.
Step 2: Convert the amount of each element in grams to its amount in moles.
Step 3: Divide each of the found values by the smallest of these values (2.7)
Step 4: If necessary, multiply these numbers by integers in order to get whole numbers; if an operation is done to one of the numbers, it must be done to all of them.
Thus, the empirical formula of methyl acetate is C
3H
6O
2. This formula also happens to be methyl acetate's molecular formula.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Empirical formula".