Empagliflozin
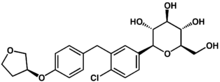 | |
|---|---|
| Systematic (IUPAC) name | |
| (2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Clinical data | |
| Legal status | Investigational |
| Identifiers | |
| CAS number | 864070-44-0 |
| ATC code | A10BX12 |
| PubChem | CID 11949646 |
| UNII | HDC1R2M35U |
| Chemical data | |
| Formula | C23H27ClO7 |
| Mol. mass | 450.91 g/mol |
| SMILES
| |
| |
Empagliflozin is drug which is being investigated in clinical trials for the oral treatment of type 2 diabetes by Boehringer Ingelheim and Eli Lilly and Company.[1][2] It is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), which is found almost exclusively in the proximal tubules of nephronic components in the kidneys. SGLT-2 accounts for about 90 percent of glucose reabsorption into the blood. Blocking SGLT-2 causes blood glucose to be eliminated through the urine via the urethra.[3][4]
Mode of action
SGLT-2 inhibitors such as empagliflozin reduce blood glucose by blocking glucose reabsorption in the kidney and thereby excreting glucose (i.e., blood sugar) via the urine.[5]
Clinical trials
As of December 2013, empagliflozin had completed some phase III clinical trials and was enrolling others.[2]
Side effects
When taken in dosages of 10 or 25 mg once a day, the incidence of adverse events was similar to placebo. However, there was a higher frequency of genital infections at both the 10 mg and the 25 mg dosages.[6]
Regulatory status
As of May 2013, Boehringer and Lilly had submitted applications for marketing approval to the European Medicines Agency and the FDA.[6]
References
- ↑ Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P (January 2012). "Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors". Diabetes Obes Metab 14 (1): 83–90. doi:10.1111/j.1463-1326.2011.01517.x. PMID 21985634.
- ↑ 2.0 2.1 "Empagliflozin". clinicaltrials.gov. U.S. National Institutes of Health. Retrieved 22 September 2012.
- ↑ Nair S, Wilding JP (January 2010). "Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus". J. Clin. Endocrinol. Metab. 95 (1): 34–42. doi:10.1210/jc.2009-0473. PMID 19892839.
- ↑ Bays H (March 2009). "From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus". Curr Med Res Opin 25 (3): 671–81. doi:10.1185/03007990802710422. PMID 19232040.
- ↑ Abdul-Ghani MA, DeFronzo RA (September 2008). "Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus". Endocr Pract 14 (6): 782–90. PMID 18996802.
- ↑ 6.0 6.1 Miriam E. Tucker for Medscape Medical News. May 07, 2013 First Details of Empagliflozin Trials Follow US and EU Filings
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||