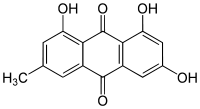
Emodin
| Emodin | |
|---|---|
 | |
 | |
| IUPAC name 1,3,8-trihydroxy-6-methylanthracene-9,10-dione | |
| Other names 6-methyl-1,3,8-trihydroxyanthraquinone | |
| Identifiers | |
| CAS number | 518-82-1 |
| PubChem | 3220 |
| ChemSpider | 3107 |
| UNII | KA46RNI6HN |
| DrugBank | DB07715 |
| KEGG | C10343 |
| ChEBI | CHEBI:42223 |
| ChEMBL | CHEMBL289277 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C15H10O5 |
| Molar mass | 270.24 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Emodin (from Rheum emodi, a Himalayan rhubarb) is a purgative resin, 6-methyl-1,3,8-trihydroxyanthraquinone, from rhubarb, buckthorn and Japanese knotweed (Fallopia japonica syn. Polygonum cuspidatum).[1] It can also be isomers in botanical extracts.
Pharmacology
Emodin is being studied as a potential agent that could reduce the impact of type 2 diabetes. It is a potent selective inhibitor of the enzyme 11β-HSD1.[2] In studies in obese mice, emodin limits the effect of glucocorticoids and may therefore ameliorate diabetes and insulin resistance.[3]
Pharmacological studies have demonstrated that emodin when isolated from rhubarb exhibits anti-cancer effects on several human cancers, including human pancreatic cancer.[4][5][6] Emodin in rhubarb extracts may also have neuroprotective properties against glutamate toxicity.[7]
Aloe-emodin (1,3,8-trihydroxyanthraquinone) is a variety of emodin found in Socotrine, Barbados, and Zanzibar aloes, but not in Natal aloes.[citation needed]
Emodin is also shown to block cytomegalovirus infections as well as herpes simplex. Research is currently being performed in this area.
List of plants that contain the chemical
- Senna obtusifolia[8] (syn. Cassia obtusifolia[9])
- Fallopia japonica[10] (syn. Polygonum cuspidatum[11])
- Ventilago madraspatana[12]
- Kalimeris indica[13]
- Rumex nepalensis[14]
- Polygonum hypoleucum[15]
- Cassia occidentalis[16]
- Cassia siamea[17]
- Acalypha australis[18]
- Rheum palmatum[19]
Compendial status
References
- ↑ Dorland's Medical Dictionary (1938)
- ↑ Feng, Y.; Huang, S. L.; Dou, W.; Zhang, S.; Chen, J. H.; Shen, Y.; Shen, J. H.; Leng, Y. (2010). "Emodin, a Natural Product, Selectively Inhibits 11β-Hydroxysteroid Dehydrogenase Type 1 and Ameliorates Metabolic Disorder in Diet-Induced Obese Mice". British Journal of Pharmacology 161 (1): 113–126. doi:10.1111/j.1476-5381.2010.00826.x. PMC 2962821. PMID 20718744.
- ↑ Novel diabetes hope comes from Chinese herbs, esciencenews.com, August 17, 2010
- ↑ Lin, S.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. (2011). "Antiproliferative and Antimetastatic Effects of Emodin on Human Pancreatic Cancer". Oncology Reports 26 (1): 81–89. doi:10.3892/or.2011.1257. PMID 21491088.
- ↑ Sun, Y. (2008). "Chemosensitization by Emodin, a Plant-Derived Anti-Cancer Agent: Mechanism of Action". Cancer Biology & Therapy 7 (3): 476–478. doi:10.4161/cbt.7.3.5584. PMID 18245955.
- ↑ Dorsey, J. F.; Kao, G. D. (2007). "Aloe(-Emodin) for Cancer? More than Just a Comforting Salve" (pdf). Cancer Biology & Therapy 6 (1): 89–90. doi:10.4161/cbt.6.1.3845. PMID 17297301.
- ↑ Gu, J. W.; Hasuo, H.; Takeya, M.; Akasu, T. (2005). "Effects of Emodin on Synaptic Transmission in Rat Hippocampal CA1 Pyramidal Neurons in vitro". Neuropharmacology 49 (1): 103–111. doi:10.1016/j.neuropharm.2005.02.003. PMID 15992585.
- ↑ Dr. Duke's Phytochemical and Ethnobotanical Databases
- ↑ Yang, Y.-C.; Lim, M.-Y.; Lee, H.-S. (2003). "Emodin Isolated from Cassia obtusifolia (Leguminosae) Seed Shows Larvicidal Activity against Three Mosquito Species". Journal of Agricultural and Food Chemistry 51 (26): 7629–7631. doi:10.1021/jf034727t. PMID 14664519.
- ↑ http://www.ars-grin.gov/cgi-bin/duke/highchem.pl
- ↑ Ban, S. H.; Kwon, Y. R.; Pandit, S.; Lee, Y. S.; Yi, H. K.; Jeon, J. G. (2010). "Effects of a Bio-Assay Guided Fraction from Polygonum cuspidatum Root on the Viability, Acid Production and Glucosyltranferase of mutans streptococci". Fitoterapia 81 (1): 30–34. doi:10.1016/j.fitote.2009.06.019. PMID 19616082.
- ↑ Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. (2010). "Anti-Inflammatory and Anticancer Compounds Isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.". Journal of Pharmacy & Pharmacology 62 (9): 1158–1166. doi:10.1111/j.2042-7158.2010.01151.x. PMID 20796195.
- ↑ Wang, G.; Wang, G. K.; Liu, J. S.; Yu, B.; Wang, F.; Liu, J. K. (2010). "[Studies on the chemical constituents of Kalimeris indica]" [Studies on the Chemical Constituents of Kalimeris indica]. Zhong Yao Cai (in Chinese) 33 (4): 551–554. PMID 20845783.
- ↑ Gautam, R.; Karkhile, K. V.; Bhutani, K. K.; Jachak, S. M. (2010). "Anti-Inflammatory, Cyclooxygenase (COX)-2, COX-1 Inhibitory, and Free Radical Scavenging Effects of Rumex nepalensis". Planta Medica 76 (14): 1564–1569. doi:10.1055/s-0030-1249779. PMID 20379952.
- ↑ Chao, P. M.; Kuo, Y. H.; Lin, Y. S.; Chen, C. H.; Chen, S. W.; Kuo, Y. H. (2010). "The Metabolic Benefits of Polygonum hypoleucum Ohwi in HepG2 Cells and Wistar Rats under Lipogenic Stress". Journal of Agricultural and Food Chemistry 58 (8): 5174–5180. doi:10.1021/jf100046h. PMID 20230058.
- ↑ Yadav, J. P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. (2010). "Cassia occidentalis L.: A Review on its Ethnobotany, Phytochemical and Pharmacological Profile". Fitoterapia 81 (4): 223–230. doi:10.1016/j.fitote.2009.09.008. PMID 19796670.
- ↑ Nsonde Ntandou, G. F.; Banzouzi, J. T.; Mbatchi, B.; Elion-Itou, R. D.; Etou-Ossibi, A. W.; Ramos, S.; Benoit-Vical, F.; Abena, A. A.; Ouamba, J. M. (2010). "Analgesic and Anti-Inflammatory Effects of Cassia siamea Lam. Stem Bark Extracts". Journal of Ethnopharmacology 127 (1): 108–111. doi:10.1016/j.jep.2009.09.040. PMID 19799981.
- ↑ Wang, X. L.; Yu, K. B.; Peng, S. L. (2008). "[Chemical constituents of aerial part of Acalypha australis]" [Chemical Constituents of Aerial Part of Acalypha australis]. Zhongguo Zhong Yao Za Zhi [China Journal of Chinese Materia Medica] (in Chinese) 33 (12): 1415–1417. PMID 18837345.
- ↑ Liu, A.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. (2011). "Antiproliferative and Antimetastatic Effects of Emodin on Human Pancreatic Cancer". Oncology Reports 26 (1): 81–89. doi:10.3892/or.2011.1257. PMID 21491088.
- ↑ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009". Retrieved 20 April 2010.
| ||||||||||||||