Elbs persulfate oxidation
From Wikipedia, the free encyclopedia
The Elbs persulfate oxidation is the organic reaction of phenols with alkaline potassium persulfate to form para-diphenols.[1]

Several reviews have been published.[2][3][4]
Reaction mechanism
A reaction mechanism has been postulated accounting for the observed para substitution featuring the tautomeric para carbanion of the starting phenolate ion:[5]
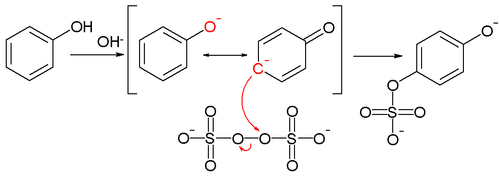
which gives a nucleophilic displacement on the peroxide oxygen of the peroxodisulfate (peroxydisulfate) ion. The intermediate sulfate group is then hydrolyzed to the hydroxyl group.
The reaction is disadvantaged by low chemical yields with recovery of starting material and complete consumption of the persulfate. It is suggested that the phenol in many cases is a catalyst converting the persulfate into a sulfate.
References
- ↑ Elbs, K. (1893). "Ueber Nitrohydrochinon". J. Prakt. Chem. 48: 179. doi:10.1002/prac.18930480123.
- ↑ Sethna, S. M. (1951). "The Elbs Persulfate Oxidation". Chem. Rev. 49 (1): 91–101. doi:10.1021/cr60152a002.
- ↑ Lee, J. B.; Uff, B. C. (1967). "Organic reactions involving electrophilic oxygen". Quart. Rev. 21 (4): 453. doi:10.1039/qr9672100429.
- ↑ Behrman, E. J. (1988). "The Persulfate Oxidation of Phenols and Arylamines (The Elbs and the Boyland-Sims Oxidations)". Org. React. 35. pp. 421–511. doi:10.1002/0471264180.or035.02. ISBN 0471264180.
- ↑ Behrman, E. J. (2006). "The Elbs and Boyland-Sims peroxydisulfate oxidations". Beilstein Journal of Organic Chemistry 2 (1): 22. doi:10.1186/1860-5397-2-22. PMC 1697820. PMID 17090305.
See also
- Boyland-Sims oxidation
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.