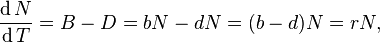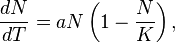Ecology
 | |
  | |
  | |
| Ecology addresses the full scale of life, from tiny bacteria to processes that span the entire planet. Ecologists study many diverse and complex relations among species, such as predation and pollination. The diversity of life is organized into different habitats, from terrestrial (middle) to aquatic ecosystems. |
Ecology (from Greek: οἶκος, "house"; -λογία, "study of"[A]) is the scientific study of interactions among organisms and their environment, such as the interactions organisms have with each other and with their abiotic environment. Topics of interest to ecologists include the diversity, distribution, amount (biomass), number (population) of organisms, as well as competition between them within and among ecosystems. Ecosystems are composed of dynamically interacting parts including organisms, the communities they make up, and the non-living components of their environment. Ecosystem processes, such as primary production, pedogenesis, nutrient cycling, and various niche construction activities, regulate the flux of energy and matter through an environment. These processes are sustained by organisms with specific life history traits, and the variety of organisms is called biodiversity. Biodiversity, which refers to the varieties of species, genes, and ecosystems, enhances certain ecosystem services.
Ecology is an interdisciplinary field that includes biology and Earth science. The word "ecology" ("Ökologie") was coined in 1866 by the German scientist Ernst Haeckel (1834–1919). Ancient Greek philosophers such as Hippocrates and Aristotle laid the foundations of ecology in their studies on natural history. Modern ecology transformed into a more rigorous science in the late 19th century. Evolutionary concepts on adaptation and natural selection became cornerstones of modern ecological theory. Ecology is not synonymous with environment, environmentalism, natural history, or environmental science. It is closely related to evolutionary biology, genetics, and ethology. An understanding of how biodiversity affects ecological function is an important focus area in ecological studies. Ecologists seek to explain:
- Life processes, interactions and adaptations
- The movement of materials and energy through living communities
- The successional development of ecosystems, and
- The abundance and distribution of organisms and biodiversity in the context of the environment.
Ecology is a human science as well. There are many practical applications of ecology in conservation biology, wetland management, natural resource management (agroecology, agriculture, forestry, agroforestry, fisheries), city planning (urban ecology), community health, economics, basic and applied science, and human social interaction (human ecology). Organisms and resources compose ecosystems which, in turn, maintain biophysical feedback mechanisms that moderate processes acting on living (biotic) and nonliving (abiotic) components of the planet. Ecosystems sustain life-supporting functions and produce natural capital like biomass production (food, fuel, fiber and medicine), the regulation of climate, global biogeochemical cycles, water filtration, soil formation, erosion control, flood protection and many other natural features of scientific, historical, economic, or intrinsic value.
Integrative levels, scope, and scale of organization

The scope of ecology covers a wide array of interacting levels of organization spanning micro-level (e.g., cells) to planetary scale (e.g., biosphere) phenomena. Ecosystems, for example, contain abiotic resources and interacting life forms (i.e., individual organisms that aggregate into populations which aggregate into distinct ecological communities). Ecosystems are dynamic, they do not always follow a linear successional path, but they are always changing, sometimes rapidly and sometimes so slowly that it can take thousands of years for ecological processes to bring about certain successional stages of a forest. An ecosystem's area can vary greatly, from tiny to vast. A single tree is of little consequence to the classification of a forest ecosystem, but critically relevant to organisms living in and on it.[1] Several generations of an aphid population can exist over the lifespan of a single leaf. Each of those aphids, in turn, support diverse bacterial communities.[2] The nature of connections in ecological communities cannot be explained by knowing the details of each species in isolation, because the emergent pattern is neither revealed nor predicted until the ecosystem is studied as an integrated whole.[3] Some ecological principles, however, do exhibit collective properties where the sum of the components explain the properties of the whole, such as birth rates of a population being equal to the sum of individual births over a designated time frame.[4]
Hierarchical ecology
System behaviors must first be arrayed into different levels of organization. Behaviors corresponding to higher levels occur at slow rates. Conversely, lower organizational levels exhibit rapid rates. For example, individual tree leaves respond rapidly to momentary changes in light intensity, CO2 concentration, and the like. The growth of the tree responds more slowly and integrates these short-term changes.
The scale of ecological dynamics can operate like a closed system, such as aphids migrating on a single tree, while at the same time remain open with regard to broader scale influences, such as atmosphere or climate. Hence, ecologists classify ecosystems hierarchically by analyzing data collected from finer scale units, such as vegetation associations, climate, and soil types, and integrate this information to identify emergent patterns of uniform organization and processes that operate on local to regional, landscape, and chronological scales.
To structure the study of ecology into a conceptually manageable framework, the biological world is organized into a nested hierarchy, ranging in scale from genes, to cells, to tissues, to organs, to organisms, to species, to populations, to communities, to ecosystems, to biomes, and up to the level of the biosphere.[6] This framework forms a panarchy[7] and exhibits non-linear behaviors; this means that "effect and cause are disproportionate, so that small changes to critical variables, such as the number of nitrogen fixers, can lead to disproportionate, perhaps irreversible, changes in the system properties."[8]:14
Biodiversity
Biodiversity refers to the variety of life and its processes. It includes the variety of living organisms, the genetic differences among them, the communities and ecosystems in which they occur, and the ecological and evolutionary processes that keep them functioning, yet ever changing and adapting.
Biodiversity (an abbreviation of "biological diversity") describes the diversity of life from genes to ecosystems and spans every level of biological organization. The term has several interpretations, and there are many ways to index, measure, characterize, and represent its complex organization.[10][11][12] Biodiversity includes species diversity, ecosystem diversity, and genetic diversity and scientists are interested in the way that this diversity affects the complex ecological processes operating at and among these respective levels.[11][13][14] Biodiversity plays an important role in ecosystem services which by definition maintain and improve human quality of life.[12][15][16] Preventing species extinctions is one way to preserve biodiversity and that goal rests on techniques that preserve genetic diversity, habitat and the ability for species to migrate.[citation needed] Conservation priorities and management techniques require different approaches and considerations to address the full ecological scope of biodiversity. Natural capital that supports populations is critical for maintaining ecosystem services[17][18] and species migration (e.g., riverine fish runs and avian insect control) has been implicated as one mechanism by which those service losses are experienced.[19] An understanding of biodiversity has practical applications for species and ecosystem-level conservation planners as they make management recommendations to consulting firms, governments, and industry.[20]
Habitat
The habitat of a species describes the environment over which a species is known to occur and the type of community that is formed as a result.[21] More specifically, "habitats can be defined as regions in environmental space that are composed of multiple dimensions, each representing a biotic or abiotic environmental variable; that is, any component or characteristic of the environment related directly (e.g. forage biomass and quality) or indirectly (e.g. elevation) to the use of a location by the animal."[22]:745 For example, a habitat might be an aquatic or terrestrial environment that can be further categorized as a montane or alpine ecosystem. Habitat shifts provide important evidence of competition in nature where one population changes relative to the habitats that most other individuals of the species occupy. For example, one population of a species of tropical lizards (Tropidurus hispidus) has a flattened body relative to the main populations that live in open savanna. The population that lives in an isolated rock outcrop hides in crevasses where its flattened body offers a selective advantage. Habitat shifts also occur in the developmental life history of amphibians and in insects that transition from aquatic to terrestrial habitats. Biotope and habitat are sometimes used interchangeably, but the former applies to a community's environment, whereas the latter applies to a species' environment.[21][23][24]
Additionally, some species are ecosystem engineers, altering the environment within a localized region. For instance, beavers manage water levels by building dams which improves their habitat in a landscape.

Niche
Definitions of the niche date back to 1917,[25] but G. Evelyn Hutchinson made conceptual advances in 1957[26][27] by introducing a widely adopted definition: "the set of biotic and abiotic conditions in which a species is able to persist and maintain stable population sizes."[25]:519 The ecological niche is a central concept in the ecology of organisms and is sub-divided into the fundamental and the realized niche. The fundamental niche is the set of environmental conditions under which a species is able to persist. The realized niche is the set of environmental plus ecological conditions under which a species persists.[25][27][28] The Hutchinsonian niche is defined more technically as a "Euclidean hyperspace whose dimensions are defined as environmental variables and whose size is a function of the number of values that the environmental values may assume for which an organism has positive fitness."[29]:71
Biogeographical patterns and range distributions are explained or predicted through knowledge of a species' traits and niche requirements.[30] Species have functional traits that are uniquely adapted to the ecological niche. A trait is a measurable property, phenotype, or characteristic of an organism that may influence its survival. Genes play an important role in the interplay of development and environmental expression of traits.[31] Resident species evolve traits that are fitted to the selection pressures of their local environment. This tends to afford them a competitive advantage and discourages similarly adapted species from having an overlapping geographic range. The competitive exclusion principle states that two species cannot coexist indefinitely by living off the same limiting resource; one will always outcompete the other. When similarly adapted species overlap geographically, closer inspection reveals subtle ecological differences in their habitat or dietary requirements.[32] Some models and empirical studies, however, suggest that disturbances can stabilize the coevolution and shared niche occupancy of similar species inhabiting species-rich communities.[33] The habitat plus the niche is called the ecotope, which is defined as the full range of environmental and biological variables affecting an entire species.[21]
Niche construction
Organisms are subject to environmental pressures, but they also modify their habitats. The regulatory feedback between organisms and their environment can affect conditions from local (e.g., a beaver pond) to global scales, over time and even after death, such as decaying logs or silica skeleton deposits from marine organisms.[34] The process and concept of ecosystem engineering has also been called niche construction. Ecosystem engineers are defined as: "organisms that directly or indirectly modulate the availability of resources to other species, by causing physical state changes in biotic or abiotic materials. In so doing they modify, maintain and create habitats."[35]:373
The ecosystem engineering concept has stimulated a new appreciation for the influence that organisms have on the ecosystem and evolutionary process. The term "niche construction" is more often used in reference to the under-appreciated feedback mechanisms of natural selection imparting forces on the abiotic niche.[36][37] An example of natural selection through ecosystem engineering occurs in the nests of social insects, including ants, bees, wasps, and termites. There is an emergent homeostasis or homeorhesis in the structure of the nest that regulates, maintains and defends the physiology of the entire colony. Termite mounds, for example, maintain a constant internal temperature through the design of air-conditioning chimneys. The structure of the nests themselves are subject to the forces of natural selection. Moreover, a nest can survive over successive generations, so that progeny inherit both genetic material and a legacy niche that was constructed before their time.[4][36][38]
Biome
Biomes are larger units of organization that categorize regions of the Earth's ecosystems, mainly according to the structure and composition of vegetation.[39] There are different methods to define the continental boundaries of biomes dominated by different functional types of vegetative communities that are limited in distribution by climate, precipitation, weather and other environmental variables. Biomes include tropical rainforest, temperate broadleaf and mixed forest, temperate deciduous forest, taiga, tundra, hot desert, and polar desert.[40] Other researchers have recently categorized other biomes, such as the human and oceanic microbiomes. To a microbe, the human body is a habitat and a landscape.[41] Microbiomes were discovered largely through advances in molecular genetics, which have revealed a hidden richness of microbial diversity on the planet. The oceanic microbiome plays a significant role in the ecological biogeochemistry of the planet's oceans.[42]
Biosphere
The largest scale of ecological organization is the biosphere: the total sum of ecosystems on the planet. Ecological relationships regulate the flux of energy, nutrients, and climate all the way up to the planetary scale. For example, the dynamic history of the planetary atmosphere's CO2 and O2 composition has been affected by the biogenic flux of gases coming from respiration and photosynthesis, with levels fluctuating over time in relation to the ecology and evolution of plants and animals.[43] Ecological theory has also been used to explain self-emergent regulatory phenomena at the planetary scale: for example, the Gaia hypothesis is an example of holism applied in ecological theory.[44] The Gaia hypothesis states that there is an emergent feedback loop generated by the metabolism of living organisms that maintains the core temperature of the Earth and atmospheric conditions within a narrow self-regulating range of tolerance.[45]
Population ecology
Population ecology studies the dynamics of specie populations and how these populations interact with the wider environment.[4] A population consists of individuals of the same species that live, interact and migrate through the same niche and habitat.[46]
A primary law of population ecology is the Malthusian growth model[47] which states, "a population will grow (or decline) exponentially as long as the environment experienced by all individuals in the population remains constant."[47]:18 Simplified population models usually start with four variables: death, birth, immigration, and emigration.
An example of an introductory population model describes a closed population, such as on an island, where immigration and emigration does not take place. Hypotheses are evaluated with reference to a null hypothesis which states that random processes create the observed data. In these island models, the rate of population change is described by:
where N is the total number of individuals in the population, B is the number of births, D is the number of deaths, b and d are the per capita rates of birth and death respectively, and r is the per capita rate of population change. The formula states that the rate of change in population size (dN/dT) is equal to births minus deaths (B – D).[47][48]
Using these modelling techniques, Malthus' population principle of growth was later transformed into a model known as the logistic equation:
where N is the number of individuals measured as biomass density, a is the maximum per-capita rate of change, and K is the carrying capacity of the population. The formula states that the rate of change in population size (dN/dT) is equal to growth (aN) that is limited by carrying capacity (1 – N/K).
Population ecology builds upon these introductory models to further understand demographic processes in real study populations. Commonly used types of data include life history, fecundity, and survivorship, and these are analysed using mathematical techniques such as matrix algebra. The information is used for managing wildlife stocks and setting harvest quotas.[48][49] In cases where basic models are insufficient, ecologists may adopt different kinds of statistical methods, such as the Akaike information criterion,[50] or use models that can become mathematically complex as "several competing hypotheses are simultaneously confronted with the data."[51]
Metapopulations and migration
The concept of metapopulations was defined in 1969[52] as "a population of populations which go extinct locally and recolonize."[53]:105 Metapopulation ecology is another statistical approach that is often used in conservation research.[54] Metapopulation models simplify the landscape into patches of varying levels of quality,[55] and metapopulations are linked by the migratory behaviours of organisms. Animal migration is set apart from other kinds of movement because it involves the seasonal departure and return of individuals from a habitat.[56] Migration is also a population-level phenomenon, as with the migration routes followed by plants as they occupied northern post-glacial environments. Plant ecologists use pollen records that accumulate and stratify in wetlands to reconstruct the timing of plant migration and dispersal relative to historic and contemporary climates. These migration routes involved an expansion of the range as plant populations expanded from one area to another. There is a larger taxonomy of movement, such as commuting, foraging, territorial behaviour, stasis, and ranging. Dispersal is usually distinguished from migration because it involves the one way permanent movement of individuals from their birth population into another population.[57][58]
In metapopulation terminology, migrating individuals are classed as emigrants (when they leave a region) or immigrants (when they enter a region), and sites are classed either as sources or sinks. A site is a generic term that refers to places where ecologists sample populations, such as ponds or defined sampling areas in a forest. Source patches are productive sites that generate a seasonal supply of juveniles that migrate to other patch locations. Sink patches are unproductive sites that only receive migrants; the population at the site will disappear unless rescued by an adjacent source patch or environmental conditions become more favourable. Metapopulation models examine patch dynamics over time to answer potential questions about spatial and demographic ecology. The ecology of metapopulations is a dynamic process of extinction and colonization. Small patches of lower quality (i.e., sinks) are maintained or rescued by a seasonal influx of new immigrants. A dynamic metapopulation structure evolves from year to year, where some patches are sinks in dry years and are sources when conditions are more favourable. Ecologists use a mixture of computer models and field studies to explain metapopulation structure.[59][60]
Community ecology

Community ecology examines how interactions among species and their environment affect the abundance, distribution and diversity of species within communities.
Community ecology is the study of the interactions among a collections of species that inhabit the same geographic area. Research in community ecology might measure primary production in a wetland in relation to decomposition and consumption rates. This requires an understanding of the community connections between plants (i.e., primary producers) and the decomposers (e.g., fungi and bacteria),[62] or the analysis of predator-prey dynamics affecting amphibian biomass.[63] Food webs and trophic levels are two widely employed conceptual models used to explain the linkages among species.[4]
Ecosystem ecology
These ecosystems, as we may call them, are of the most various kinds and sizes. They form one category of the multitudinous physical systems of the universe, which range from the universe as a whole down to the atom.

Ecosystems are habitats within biomes that form an integrated whole and a dynamically responsive system having both physical and biological complexes. The underlying concept can be traced back to 1864 in the published work of George Perkins Marsh ("Man and Nature").[65][66] Within an ecosystem, organisms are linked to the physical and biological components of their environment to which they are adapted.[64] Ecosystems are complex adaptive systems where the interaction of life processes form self-organizing patterns across different scales of time and space.[67] Ecosystems are broadly categorized as terrestrial, freshwater, atmospheric, or marine. Differences stem from the nature of the unique physical environments that shapes the biodiversity within each. A more recent addition to ecosystem ecology are technoecosystems, which are affected by or primarily the result of human activity.[4]
Food webs
A food web is the archetypal ecological network. Plants capture solar energy and use it to synthesize simple sugars during photosynthesis. As plants grow, they accumulate nutrients and are eaten by grazing herbivores, and the energy is transferred through a chain of organisms by consumption. The simplified linear feeding pathways that move from a basal trophic species to a top consumer is called the food chain. The larger interlocking pattern of food chains in an ecological community creates a complex food web. Food webs are a type of concept map or a heuristic device that is used to illustrate and study pathways of energy and material flows.[5][68][69]

Food webs are often limited relative to the real world. Complete empirical measurements are generally restricted to a specific habitat, such as a cave or a pond, and principles gleaned from food web microcosm studies are extrapolated to larger systems.[70] Feeding relations require extensive investigations into the gut contents of organisms, which can be difficult to decipher, or stable isotopes can be used to trace the flow of nutrient diets and energy through a food web.[71] Despite these limitations, food webs remain a valuable tool in understanding community ecosystems.[72]
Food webs exhibit principles of ecological emergence through the nature of trophic relationships: some species have many weak feeding links (e.g., omnivores) while some are more specialized with fewer stronger feeding links (e.g., primary predators). Theoretical and empirical studies identify non-random emergent patterns of few strong and many weak linkages that explain how ecological communities remain stable over time.[73] Food webs are composed of subgroups where members in a community are linked by strong interactions, and the weak interactions occur between these subgroups. This increases food web stability.[74] Step by step lines or relations are drawn until a web of life is illustrated.[69][75][76][77]
Trophic levels

A trophic level (from Greek troph, τροφή, trophē, meaning "food" or "feeding") is "a group of organisms acquiring a considerable majority of its energy from the adjacent level nearer the abiotic source."[78]:383 Links in food webs primarily connect feeding relations or trophism among species. Biodiversity within ecosystems can be organized into trophic pyramids, in which the vertical dimension represents feeding relations that become further removed from the base of the food chain up toward top predators, and the horizontal dimension represents the abundance or biomass at each level.[79] When the relative abundance or biomass of each species is sorted into its respective trophic level, they naturally sort into a 'pyramid of numbers'.[80]
Species are broadly categorized as autotrophs (or primary producers), heterotrophs (or consumers), and Detritivores (or decomposers). Autotrophs are organisms that produce their own food (production is greater than respiration) by photosynthesis or chemosynthesis. Heterotrophs are organisms that must feed on others for nourishment and energy (respiration exceeds production).[4] Heterotrophs can be further sub-divided into different functional groups, including primary consumers (strict herbivores), secondary consumers (carnivorous predators that feed exclusively on herbivores) and tertiary consumers (predators that feed on a mix of herbivores and predators).[81] Omnivores do not fit neatly into a functional category because they eat both plant and animal tissues. It has been suggested that omnivores have a greater functional influence as predators, because compared to herbivores they are relatively inefficient at grazing.[82]
Trophic levels are part of the holistic or complex systems view of ecosystems.[83][84] Each trophic level contains unrelated species that are grouped together because they share common ecological functions, giving a macroscopic view of the system.[85] While the notion of trophic levels provides insight into energy flow and top-down control within food webs, it is troubled by the prevalence of omnivory in real ecosystems. This has led some ecologists to "reiterate that the notion that species clearly aggregate into discrete, homogeneous trophic levels is fiction."[86]:815 Nonetheless, recent studies have shown that real trophic levels do exist, but "above the herbivore trophic level, food webs are better characterized as a tangled web of omnivores."[87]:612
Keystone species

A keystone species is a species that is connected to a disproportionately large number of other species in the food-web. Keystone species have lower levels of biomass in the trophic pyramid relative to the importance of their role. The many connections that a keystone species holds means that it maintains the organization and structure of entire communities. The loss of a keystone species results in a range of dramatic cascading effects that alters trophic dynamics, other food web connections, and can cause the extinction of other species.[88][89]
Sea otters (Enhydra lutris) are commonly cited as an example of a keystone species because they limit the density of sea urchins that feed on kelp. If sea otters are removed from the system, the urchins graze until the kelp beds disappear and this has a dramatic effect on community structure.[90] Hunting of sea otters, for example, is thought to have indirectly led to the extinction of the Steller's Sea Cow (Hydrodamalis gigas).[91] While the keystone species concept has been used extensively as a conservation tool, it has been criticized for being poorly defined from an operational stance. It is difficult to experimentally determine what species may hold a keystone role in each ecosystem. Furthermore, food web theory suggests that keystone species may not be common, so it is unclear how generally the keystone species model can be applied.[90][92]
Ecological complexity
Complexity is understood as a large computational effort needed to piece together numerous interacting parts exceeding the iterative memory capacity of the human mind. Global patterns of biological diversity are complex. This biocomplexity stems from the interplay among ecological processes that operate and influence patterns at different scales that grade into each other, such as transitional areas or ecotones spanning landscapes. Complexity stems from the interplay among levels of biological organization as energy and matter is integrated into larger units that superimpose onto the smaller parts. "What were wholes on one level become parts on a higher one."[93]:209 Small scale patterns do not necessarily explain large scale phenomena, otherwise captured in the expression (coined by Aristotle) 'the sum is greater than the parts'.[94][95][E]
"Complexity in ecology is of at least six distinct types: spatial, temporal, structural, process, behavioral, and geometric."[96]:3 From these principles, ecologists have identified emergent and self-organizing phenomena that operate at different environmental scales of influence, ranging from molecular to planetary, and these require different explanations at each integrative level.[45][97] Ecological complexity relates to the dynamic resilience of ecosystems that transition to multiple shifting steady-states directed by random fluctuations of history.[7][98] Long-term ecological studies provide important track records to better understand the complexity and resilience of ecosystems over longer temporal and broader spatial scales. These studies are managed by the International Long Term Ecological Network (LTER).[99] The longest experiment in existence is the Park Grass Experiment, which was initiated in 1856.[100] Another example is the Hubbard Brook study, which has been in operation since 1960.[101]
Holism
Holism remains a critical part of the theoretical foundation in contemporary ecological studies. Holism addresses the biological organization of life that self-organizes into layers of emergent whole systems that function according to nonreducible properties. This means that higher order patterns of a whole functional system, such as an ecosystem, cannot be predicted or understood by a simple summation of the parts.[102] "New properties emerge because the components interact, not because the basic nature of the components is changed."[4]:8
Ecological studies are necessarily holistic as opposed to reductionistic.[31][97][103] Holism has three scientific meanings or uses that identify with ecology: 1) the mechanistic complexity of ecosystems, 2) the practical description of patterns in quantitative reductionist terms where correlations may be identified but nothing is understood about the causal relations without reference to the whole system, which leads to 3) a metaphysical hierarchy whereby the causal relations of larger systems are understood without reference to the smaller parts. Scientific holism differs from mysticism that has appropriated the same term. An example of metaphysical holism is identified in the trend of increased exterior thickness in shells of different species. The reason for a thickness increase can be understood through reference to principles of natural selection via predation without need to reference or understand the biomolecular properties of the exterior shells.[104]
Relation to evolution
Ecology and evolution are considered sister disciplines of the life sciences. Natural selection, life history, development, adaptation, populations, and inheritance are examples of concepts that thread equally into ecological and evolutionary theory. Morphological, behavioural and genetic traits, for example, can be mapped onto evolutionary trees to study the historical development of a species in relation to their functions and roles in different ecological circumstances. In this framework, the analytical tools of ecologists and evolutionists overlap as they organize, classify and investigate life through common systematic principals, such as phylogenetics or the Linnaean system of taxonomy.[105] The two disciplines often appear together, such as in the title of the journal Trends in Ecology and Evolution.[106] There is no sharp boundary separating ecology from evolution and they differ more in their areas of applied focus. Both disciplines discover and explain emergent and unique properties and processes operating across different spatial or temporal scales of organization.[31][45] While the boundary between ecology and evolution is not always clear, ecologists study the abiotic and biotic factors that influence evolutionary processes,[107][108] and evolution can be rapid, occurring on ecological timescales as short as one generation.[109]
Behavioural ecology

All organisms can exhibit behaviours. Even plants express complex behaviour, including memory and communication.[110] Behavioural ecology is the study of an organism's behaviour in its environment and its ecological and evolutionary implications. Ethology is the study of observable movement or behaviour in animals. This could include investigations of motile sperm of plants, mobile phytoplankton, zooplankton swimming toward the female egg, the cultivation of fungi by weevils, the mating dance of a salamander, or social gatherings of amoeba.[111][112][113][114][115]
Adaptation is the central unifying concept in behavioural ecology.[116] Behaviours can be recorded as traits and inherited in much the same way that eye and hair colour can. Behaviours can evolve by means of natural selection as adaptive traits conferring functional utilities that increases reproductive fitness.[117][118]
Predator-prey interactions are an introductory concept into food-web studies as well as behavioural ecology.[119] Prey species can exhibit different kinds of behavioural adaptations to predators, such as avoid, flee or defend. Many prey species are faced with multiple predators that differ in the degree of danger posed. To be adapted to their environment and face predatory threats, organisms must balance their energy budgets as they invest in different aspects of their life history, such as growth, feeding, mating, socializing, or modifying their habitat. Hypotheses posited in behavioural ecology are generally based on adaptive principles of conservation, optimization or efficiency.[28][107][120] For example, "[t]he threat-sensitive predator avoidance hypothesis predicts that prey should assess the degree of threat posed by different predators and match their behaviour according to current levels of risk"[121] or "[t]he optimal flight initiation distance occurs where expected postencounter fitness is maximized, which depends on the prey's initial fitness, benefits obtainable by not fleeing, energetic escape costs, and expected fitness loss due to predation risk."[122]

Elaborate sexual displays and posturing are encountered in the behavioural ecology of animals. The birds of paradise, for example, sing and display elaborate ornaments during courtship. These displays serve a dual purpose of signalling healthy or well-adapted individuals and desirable genes. The displays are driven by sexual selection as an advertisement of quality of traits among suitors.[123]
Social ecology
Social ecological behaviours are notable in the social insects, slime moulds, social spiders, human society, and naked mole rats where eusocialism has evolved. Social behaviours include reciprocally beneficial behaviours among kin and nest mates[113][118][124] and evolve from kin and group selection. Kin selection explains altruism through genetic relationships, whereby an altruistic behaviour leading to death is rewarded by the survival of genetic copies distributed among surviving relatives. The social insects, including ants, bees and wasps are most famously studied for this type of relationship because the male drones are clones that share the same genetic make-up as every other male in the colony.[118] In contrast, group selectionists find examples of altruism among non-genetic relatives and explain this through selection acting on the group, whereby it becomes selectively advantageous for groups if their members express altruistic behaviours to one another. Groups with predominantly altruistic members beat groups with predominantly selfish members.[118][125]
Coevolution

Ecological interactions can be classified broadly into a host and an associate relationship. A host is any entity that harbours another that is called the associate.[126] Relationships within a species that are mutually or reciprocally beneficial are called mutualisms. Examples of mutualism include fungus-growing ants employing agricultural symbiosis, bacteria living in the guts of insects and other organisms, the fig wasp and yucca moth pollination complex, lichens with fungi and photosynthetic algae, and corals with photosynthetic algae.[127][128] If there is a physical connection between host and associate, the relationship is called symbiosis. Approximately 60% of all plants, for example, have a symbiotic relationship with arbuscular mycorrhizal fungi living in their roots forming an exchange network of carbohydrates for mineral nutrients.[129]
Indirect mutualisms occur where the organisms live apart. For example, trees living in the equatorial regions of the planet supply oxygen into the atmosphere that sustains species living in distant polar regions of the planet. This relationship is called commensalism because many others receive the benefits of clean air at no cost or harm to trees supplying the oxygen.[4][130] If the associate benefits while the host suffers, the relationship is called parasitism. Although parasites impose a cost to their host (e.g., via damage to their reproductive organs or propagules, denying the services of a beneficial partner), their net effect on host fitness is not necessarily negative and, thus, becomes difficult to forecast.[131][132] Coevolution is also driven by competition among species or among members of the same species under the banner of reciprocal antagonism, such as grasses competing for growth space. The Red Queen Hypothesis, for example, posits that parasites track down and specialize on the locally common genetic defence systems of its host that drives the evolution of sexual reproduction to diversify the genetic constituency of populations responding to the antagonistic pressure.[133][134]

Biogeography
Biogeography (an amalgamation of biology and geography) is the comparative study of the geographic distribution of organisms and the corresponding evolution of their traits in space and time.[135] The Journal of Biogeography was established in 1974.[136] Biogeography and ecology share many of their disciplinary roots. For example, the theory of island biogeography, published by the mathematician Robert MacArthur and ecologist Edward O. Wilson in 1967[137] is considered one of the fundamentals of ecological theory.[138]
Biogeography has a long history in the natural sciences concerning the spatial distribution of plants and animals. Ecology and evolution provide the explanatory context for biogeographical studies.[135] Biogeographical patterns result from ecological processes that influence range distributions, such as migration and dispersal.[138] and from historical processes that split populations or species into different areas. The biogeographic processes that result in the natural splitting of species explains much of the modern distribution of the Earth's biota. The splitting of lineages in a species is called vicariance biogeography and it is a sub-discipline of biogeography.[139] There are also practical applications in the field of biogeography concerning ecological systems and processes. For example, the range and distribution of biodiversity and invasive species responding to climate change is a serious concern and active area of research in the context of global warming.[140][141]
r/K-Selection theory
A population ecology concept is r/K selection theory,[D] one of the first predictive models in ecology used to explain life-history evolution. The premise behind the r/K selection model is that natural selection pressures change according to population density. For example, when an island is first colonized, density of individuals is low. The initial increase in population size is not limited by competition, leaving an abundance of available resources for rapid population growth. These early phases of population growth experience density-independent forces of natural selection, which is called r-selection. As the population becomes more crowded, it approaches the island's carrying capacity, thus forcing individuals to compete more heavily for fewer available resources. Under crowded conditions, the population experiences density-dependent forces of natural selection, called K-selection.[142]
In the r/K-selection model, the first variable r is the intrinsic rate of natural increase in population size and the second variable K is the carrying capacity of a population.[28] Different species evolve different life-history strategies spanning a continuum between these two selective forces. An r-selected species is one that has high birth rates, low levels of parental investment, and high rates of mortality before individuals reach maturity. Evolution favours high rates of fecundity in r-selected species. Many kinds of insects and invasive species exhibit r-selected characteristics. In contrast, a K-selected species has low rates of fecundity, high levels of parental investment in the young, and low rates of mortality as individuals mature. Humans and elephants are examples of species exhibiting K-selected characteristics, including longevity and efficiency in the conversion of more resources into fewer offspring.[137][143]
Molecular ecology
The important relationship between ecology and genetic inheritance predates modern techniques for molecular analysis. Molecular ecological research became more feasible with the development of rapid and accessible genetic technologies, such as the polymerase chain reaction (PCR). The rise of molecular technologies and influx of research questions into this new ecological field resulted in the publication Molecular Ecology in 1992.[144] Molecular ecology uses various analytical techniques to study genes in an evolutionary and ecological context. In 1994, John Avise also played a leading role in this area of science with the publication of his book, Molecular Markers, Natural History and Evolution.[145] Newer technologies opened a wave of genetic analysis into organisms once difficult to study from an ecological or evolutionary standpoint, such as bacteria, fungi and nematodes. Molecular ecology engendered a new research paradigm for investigating ecological questions considered otherwise intractable. Molecular investigations revealed previously obscured details in the tiny intricacies of nature and improved resolution into probing questions about behavioural and biogeographical ecology.[145] For example, molecular ecology revealed promiscuous sexual behaviour and multiple male partners in tree swallows previously thought to be socially monogamous.[146] In a biogeographical context, the marriage between genetics, ecology and evolution resulted in a new sub-discipline called phylogeography.[147]
Human ecology
The history of life on Earth has been a history of interaction between living things and their surroundings. To a large extent, the physical form and the habits of the earth's vegetation and its animal life have been molded by the environment. Considering the whole span of earthly time, the opposite effect, in which life actually modifies its surroundings, has been relatively slight. Only within the moment of time represented by the present century has one species man acquired significant power to alter the nature of his world.
Ecology is as much a biological science as it is a human science.[4] Human ecology is an interdisciplinary investigation into the ecology of our species. "Human ecology may be defined: (1) from a bio-ecological standpoint as the study of man as the ecological dominant in plant and animal communities and systems; (2) from a bio-ecological standpoint as simply another animal affecting and being affected by his physical environment; and (3) as a human being, somehow different from animal life in general, interacting with physical and modified environments in a distinctive and creative way. A truly interdisciplinary human ecology will most likely address itself to all three."[149]:3 The term was formally introduced in 1921, but many sociologists, geographers, psychologists, and other disciplines were interested in human relations to natural systems centuries prior, especially in the late 19th century.[149][150]
The ecological complexities human beings are facing through the technological transformation of the planetary biome has brought on the Anthropocene. The unique set of circumstances has generated the need for a new unifying science called coupled human and natural systems that builds upon, but moves beyond the field of human ecology.[102] Ecosystems tie into human societies through the critical and all encompassing life-supporting functions they sustain. In recognition of these functions and the incapability of traditional economic valuation methods to see the value in ecosystems, there has been a surge of interest in social-natural capital, which provides the means to put a value on the stock and use of information and materials stemming from ecosystem goods and services. Ecosystems produce, regulate, maintain, and supply services of critical necessity and beneficial to human health (cognitive and physiological), economies, and they even provide an information or reference function as a living library giving opportunities for science and cognitive development in children engaged in the complexity of the natural world. Ecosystems relate importantly to human ecology as they are the ultimate base foundation of global economics as every commodity and the capacity for exchange ultimately stems from the ecosystems on Earth.[102][151][152][153]
Restoration and management
Ecosystem management is not just about science nor is it simply an extension of traditional resource management; it offers a fundamental reframing of how humans may work with nature.
Ecology is an employed science of restoration, repairing disturbed sites through human intervention, in natural resource management, and in environmental impact assessments. Edward O. Wilson predicted in 1992 that the 21st century "will be the era of restoration in ecology".[155] Ecological science has boomed in the industrial investment of restoring ecosystems and their processes in abandoned sites after disturbance. Natural resource managers, in forestry, for example, employ ecologists to develop, adapt, and implement ecosystem based methods into the planning, operation, and restoration phases of land-use. Ecological science is used in the methods of sustainable harvesting, disease and fire outbreak management, in fisheries stock management, for integrating land-use with protected areas and communities, and conservation in complex geo-political landscapes.[20][154][154][156][157]
Relation to the environment
The environment of ecosystems includes both physical parameters and biotic attributes. It is dynamically interlinked, and contains resources for organisms at any time throughout their life cycle.[4][158] Like "ecology," the term "environment" has different conceptual meanings and overlaps with the concept of "nature." Environment "... includes the physical world, the social world of human relations and the built world of human creation."[159]:62 The physical environment is external to the level of biological organization under investigation, including abiotic factors such as temperature, radiation, light, chemistry, climate and geology. The biotic environment includes genes, cells, organisms, members of the same species (conspecifics) and other species that share a habitat.[160]
The distinction between external and internal environments, however, is an abstraction parsing life and environment into units or facts that are inseparable in reality. There is an interpenetration of cause and effect between the environment and life. The laws of thermodynamics, for example, apply to ecology by means of its physical state. With an understanding of metabolic and thermodynamic principles, a complete accounting of energy and material flow can be traced through an ecosystem. In this way, the environmental and ecological relations are studied through reference to conceptually manageable and isolated material parts. After the effective environmental components are understood through reference to their causes, however, they conceptually link back together as an integrated whole, or holocoenotic system as it was once called. This is known as the dialectical approach to ecology. The dialectical approach examines the parts, but integrates the organism and the environment into a dynamic whole (or umwelt). Change in one ecological or environmental factor can concurrently affect the dynamic state of an entire ecosystem.[31][161]
Disturbance and resilience
Ecosystems are regularly confronted with natural environmental variations and disturbances over time and geographic space. A disturbance is any process that removes biomass from a community, such as a fire, flood, drought, or predation.[162] Disturbances occur over vastly different ranges in terms of magnitudes as well as distances and time periods,[163] and are both the cause and product of natural fluctuations in death rates, species assemblages, and biomass densities within an ecological community. These disturbances create places of renewal where new directions emerge from the patchwork of natural experimentation and opportunity.[162][164][165] Ecological resilience is a cornerstone theory in ecosystem management. Biodiversity fuels the resilience of ecosystems acting as a kind of regenerative insurance.[165]
Metabolism and the early atmosphere
Metabolism – the rate at which energy and material resources are taken up from the environment, transformed within an organism, and allocated to maintenance, growth and reproduction – is a fundamental physiological trait.
The Earth was formed approximately 4.5 billion years ago.[167] As it cooled and a crust and oceans formed, its atmosphere transformed from being dominated by hydrogen to one composed mostly of methane and ammonia. Over the next billion years, the metabolic activity of life transformed the atmosphere into a mixture of carbon dioxide, nitrogen, and water vapor. These gases changed the way that light from the sun hit the Earth's surface and greenhouse effects trapped heat. There were untapped sources of free energy within the mixture of reducing and oxidizing gasses that set the stage for primitive ecosystems to evolve and, in turn, the atmosphere also evolved.[168]

Throughout history, the Earth's atmosphere and biogeochemical cycles have been in a dynamic equilibrium with planetary ecosystems. The history is characterized by periods of significant transformation followed by millions of years of stability.[169] The evolution of the earliest organisms, likely anaerobic methanogen microbes, started the process by converting atmospheric hydrogen into methane (4H2 + CO2 → CH4 + 2H2O). Anoxygenic photosynthesis reduced hydrogen concentrations and increased atmospheric methane, by converting hydrogen sulfide into water or other sulfur compounds (for example, 2H2S + CO2 + hv → CH2O + H2O + 2S). Early forms of fermentation also increased levels of atmospheric methane. The transition to an oxygen-dominant atmosphere (the Great Oxidation) did not begin until approximately 2.4–2.3 billion years ago, but photosynthetic processes started 0.3 to 1 billion years prior.[169][170]
Radiation: heat, temperature and light
The biology of life operates within a certain range of temperatures. Heat is a form of energy that regulates temperature. Heat affects growth rates, activity, behaviour and primary production. Temperature is largely dependent on the incidence of solar radiation. The latitudinal and longitudinal spatial variation of temperature greatly affects climates and consequently the distribution of biodiversity and levels of primary production in different ecosystems or biomes across the planet. Heat and temperature relate importantly to metabolic activity. Poikilotherms, for example, have a body temperature that is largely regulated and dependent on the temperature of the external environment. In contrast, homeotherms regulate their internal body temperature by expending metabolic energy.[107][108][161]
There is a relationship between light, primary production, and ecological energy budgets. Sunlight is the primary input of energy into the planet's ecosystems. Light is composed of electromagnetic energy of different wavelengths. Radiant energy from the sun generates heat, provides photons of light measured as active energy in the chemical reactions of life, and also acts as a catalyst for genetic mutation.[107][108][161] Plants, algae, and some bacteria absorb light and assimilate the energy through photosynthesis. Organisms capable of assimilating energy by photosynthesis or through inorganic fixation of H2S are autotrophs. Autotrophs — responsible for primary production — assimilate light energy which becomes metabolically stored as potential energy in the form of biochemical enthalpic bonds.[107][108][161]
Physical environments
Water
Wetland conditions such as shallow water, high plant productivity, and anaerobic substrates provide a suitable environment for important physical, biological, and chemical processes. Because of these processes, wetlands play a vital role in global nutrient and element cycles.
Diffusion of carbon dioxide and oxygen is approximately 10,000 times slower in water than in air. When soils are flooded, they quickly lose oxygen, becoming hypoxic (an environment with O2 concentration below 2 mg/liter) and eventually completely anoxic where anaerobic bacteria thrive among the roots. Water also influences the intensity and spectral composition of light as it reflects off the water surface and submerged particles.[171] Aquatic plants exhibit a wide variety of morphological and physiological adaptations that allow them to survive, compete and diversify in these environments. For example, their roots and stems contain large air spaces (aerenchyma) that regulate the efficient transportation of gases (for example, CO2 and O2) used in respiration and photosynthesis. Salt water plants (halophytes) have additional specialized adaptations, such as the development of special organs for shedding salt and osmoregulating their internal salt (NaCl) concentrations, to live in estuarine, brackish, or oceanic environments. Anaerobic soil microorganisms in aquatic environments use nitrate, manganese ions, ferric ions, sulfate, carbon dioxide and some organic compounds; other microorganisms are facultative anaerobes and use oxygen during respiration when the soil becomes drier. The activity of soil microorganisms and the chemistry of the water reduces the oxidation-reduction potentials of the water. Carbon dioxide, for example, is reduced to methane (CH4) by methanogenic bacteria.[171] The physiology of fish is also specially adapted to compensate for environmental salt levels through osmoregulation. Their gills form electrochemical gradients that mediate salt excretion in salt water and uptake in fresh water.[172]
Gravity
The shape and energy of the land is significantly affected by gravitational forces. On a large scale, the distribution of gravitational forces on the earth is uneven and influences the shape and movement of tectonic plates as well as influencing geomorphic processes such as orogeny and erosion. These forces govern many of the geophysical properties and distributions of ecological biomes across the Earth. On the organismal scale, gravitational forces provide directional cues for plant and fungal growth (gravitropism), orientation cues for animal migrations, and influence the biomechanics and size of animals.[107] Ecological traits, such as allocation of biomass in trees during growth are subject to mechanical failure as gravitational forces influence the position and structure of branches and leaves.[173] The cardiovascular systems of animals are functionally adapted to overcome pressure and gravitational forces that change according to the features of organisms (e.g., height, size, shape), their behaviour (e.g., diving, running, flying), and the habitat occupied (e.g., water, hot deserts, cold tundra).[174]
Pressure
Climatic and osmotic pressure places physiological constraints on organisms, especially those that fly and respire at high altitudes, or dive to deep ocean depths. These constraints influence vertical limits of ecosystems in the biosphere, as organisms are physiologically sensitive and adapted to atmospheric and osmotic water pressure differences.[107] For example, oxygen levels decrease with decreasing pressure and are a limiting factor for life at higher altitudes.[175] Water transportation by plants is another important ecophysiological parameter affected by osmotic pressure gradients.[176][177][178] Water pressure in the depths of oceans requires that organisms adapt to these conditions. For example, diving animals such as whales, dolphins and seals are specially adapted to deal with changes in sound due to water pressure differences.[179] Differences between hagfish species provide another example of adaptation to deep-sea pressure through specialized protein adaptations.[180]
Wind and turbulence

Turbulent forces in air and water affect the environment and ecosystem distribution, form and dynamics. On a planetary scale, ecosystems are affected by circulation patterns in the global trade winds. Wind power and the turbulent forces it creates can influence heat, nutrient, and biochemical profiles of ecosystems.[107] For example, wind running over the surface of a lake creates turbulence, mixing the water column and influencing the environmental profile to create thermally layered zones, affecting how fish, algae, and other parts of the aquatic ecosystem are structured.[181][182] Wind speed and turbulence also influence evapotranspiration rates and energy budgets in plants and animals.[171][183] Wind speed, temperature and moisture content can vary as winds travel across different land features and elevations. For example, the westerlies come into contact with the coastal and interior mountains of western North America to produce a rain shadow on the leeward side of the mountain. The air expands and moisture condenses as the winds increase in elevation; this is called orographic lift and can cause precipitation. This environmental process produces spatial divisions in biodiversity, as species adapted to wetter conditions are range-restricted to the coastal mountain valleys and unable to migrate across the xeric ecosystems (e.g., of the Columbia Basin in western North America) to intermix with sister lineages that are segregated to the interior mountain systems.[184][185]
Fire


Plants convert carbon dioxide into biomass and emit oxygen into the atmosphere. By approximately 350 million years ago (the end of the Devonian period), photosynthesis had brought the concentration of atmospheric oxygen above 17%, which allowed combustion to occur.[186] Fire releases CO2 and converts fuel into ash and tar. Fire is a significant ecological parameter that raises many issues pertaining to its control and suppression.[187] While the issue of fire in relation to ecology and plants has been recognized for a long time,[188] Charles Cooper brought attention to the issue of forest fires in relation to the ecology of forest fire suppression and management in the 1960s.[189][190]
Native North Americans were among the first to influence fire regimes by controlling their spread near their homes or by lighting fires to stimulate the production of herbaceous foods and basketry materials.[191] Fire creates a heterogenous ecosystem age and canopy structure, and the altered soil nutrient supply and cleared canopy structure opens new ecological niches for seedling establishment.[192][193] Most ecosystems are adapted to natural fire cycles. Plants, for example, are equipped with a variety of adaptations to deal with forest fires. Some species (e.g., Pinus halepensis) cannot germinate until after their seeds have lived through a fire or been exposed to certain compounds from smoke. Environmentally triggered germination of seeds is called serotiny.[194][195] Fire plays a major role in the persistence and resilience of ecosystems.[164]
Soils
Soil is the living top layer of mineral and organic dirt that covers the surface of the planet. It is the chief organizing centre of most ecosystem functions, and it is of critical importance in agricultural science and ecology. The decomposition of dead organic matter (for example, leaves on the forest floor), results in soils containing minerals and nutrients that feed into plant production. The whole of the planet's soil ecosystems is called the pedosphere where a large biomass of the Earth's biodiversity organizes into trophic levels. Invertebrates that feed and shred larger leaves, for example, create smaller bits for smaller organisms in the feeding chain. Collectively, these organisms are the detritivores that regulate soil formation.[196][197] Tree roots, fungi, bacteria, worms, ants, beetles, centipedes, spiders, mammals, birds, reptiles, amphibians and other less familiar creatures all work to create the trophic web of life in soil ecosystems. Soils form composite phenotypes where inorganic matter is enveloped into the physiology of a whole community. As organisms feed and migrate through soils they physically displace materials, an ecological process called bioturbation. This aerates soils and stimulates heterotrophic growth and production. Soil microorganisms are influenced by and feed back into the trophic dynamics of the ecosystem. No single axis of causality can be discerned to segregate the biological from geomorphological systems in soils.[198][199] Paleoecological studies of soils places the origin for bioturbation to a time before the Cambrian period. Other events, such as the evolution of trees and the colonization of land in the Devonian period played a significant role in the early development of ecological trophism in soils.[63][197][200]
Biogeochemistry and climate
Ecologists study and measure nutrient budgets to understand how these materials are regulated, flow, and recycled through the environment.[107][108][161] This research has led to an understanding that there is global feedback between ecosystems and the physical parameters of this planet, including minerals, soil, pH, ions, water and atmospheric gases. Six major elements (hydrogen, carbon, nitrogen, oxygen, sulfur, and phosphorus; H, C, N, O, S, and P) form the constitution of all biological macromolecules and feed into the Earth's geochemical processes. From the smallest scale of biology, the combined effect of billions upon billions of ecological processes amplify and ultimately regulate the biogeochemical cycles of the Earth. Understanding the relations and cycles mediated between these elements and their ecological pathways has significant bearing toward understanding global biogeochemistry.[201]
The ecology of global carbon budgets gives one example of the linkage between biodiversity and biogeochemistry. It is estimated that the Earth's oceans hold 40,000 gigatonnes (Gt) of carbon, that vegetation and soil hold 2070 Gt, and that fossil fuel emissions are 6.3 Gt carbon per year.[202] There have been major restructurings in these global carbon budgets during the Earth's history, regulated to a large extent by the ecology of the land. For example, through the early-mid Eocene volcanic outgassing, the oxidation of methane stored in wetlands, and seafloor gases increased atmospheric CO2 (carbon dioxide) concentrations to levels as high as 3500 ppm.[203]
In the Oligocene, from 25 to 32 million years ago, there was another significant restructuring of the global carbon cycle as grasses evolved a new mechanism of photosynthesis, C4 photosynthesis, and expanded their ranges. This new pathway evolved in response to the drop in atmospheric CO2 concentrations below 550 ppm.[204] The relative abundance and distribution of biodiversity alters the dynamics between organisms and their environment such that ecosystems can be both cause and effect in relation to climate change. Human-driven modifications to the planet's ecosystems (e.g., disturbance, biodiversity loss, agriculture) contributes to rising atmospheric greenhouse gas levels. Transformation of the global carbon cycle in the next century is projected to raise planetary temperatures, lead to more extreme fluctuations in weather, alter species distributions, and increase extinction rates. The effect of global warming is already being registered in melting glaciers, melting mountain ice caps, and rising sea levels. Consequently, species distributions are changing along waterfronts and in continental areas where migration patterns and breeding grounds are tracking the prevailing shifts in climate. Large sections of permafrost are also melting to create a new mosaic of flooded areas having increased rates of soil decomposition activity that raises methane (CH4) emissions. There is concern over increases in atmospheric methane in the context of the global carbon cycle, because methane is a greenhouse gas that is 23 times more effective at absorbing long-wave radiation than CO2 on a 100-year time scale.[205] Hence, there is a relationship between global warming, decomposition and respiration in soils and wetlands producing significant climate feedbacks and globally altered biogeochemical cycles.[102][206][207][208][209][210]
History
Early beginnings
Ecology has a complex origin, due in large part to its interdisciplinary nature.[211] Ancient Greek philosophers such as Hippocrates and Aristotle were among the first to record observations on natural history. However, they viewed life in terms of essentialism, where species were conceptualized as static unchanging things while varieties were seen as aberrations of an idealized type. This contrasts against the modern understanding of ecological theory where varieties are viewed as the real phenomena of interest and having a role in the origins of adaptations by means of natural selection.[4][212][213] Early conceptions of ecology, such as a balance and regulation in nature can be traced to Herodotus (died c. 425 BC), who described one of the earliest accounts of mutualism in his observation of "natural dentistry". Basking Nile crocodiles, he noted, would open their mouths to give sandpipers safe access to pluck leeches out, giving nutrition to the sandpiper and oral hygiene for the crocodile.[211] Aristotle was an early influence on the philosophical development of ecology. He and his student Theophrastus made extensive observations on plant and animal migrations, biogeography, physiology, and on their behaviour, giving an early analogue to the modern concept of an ecological niche.[214][215]
Ecological concepts such as food chains, population regulation, and productivity were first developed in the 1700s, through the published works of microscopist Antoni van Leeuwenhoek (1632–1723) and botanist Richard Bradley (1688?–1732).[4] Biogeographer Alexander von Humboldt (1769–1859) was an early pioneer in ecological thinking and was among the first to recognize ecological gradients, where species are replaced or altered in form along environmental gradients, such as a cline forming along a rise in elevation. Humboldt drew inspiration from Isaac Newton as he developed a form of "terrestrial physics." In Newtonian fashion, he brought a scientific exactitude for measurement into natural history and even alluded to concepts that are the foundation of a modern ecological law on species-to-area relationships.[216][217][218] Natural historians, such as Humboldt, James Hutton and Jean-Baptiste Lamarck (among others) laid the foundations of the modern ecological sciences.[219] The term "ecology" (German: Oekologie, Ökologie) is of a more recent origin and was first coined by the German biologist Ernst Haeckel in his book Generelle Morphologie der Organismen (1866). Haeckel was a zoologist, artist, writer, and later in life a professor of comparative anatomy.[220][221]
By ecology, we mean the whole science of the relations of the organism to the environment including, in the broad sense, all the "conditions of existence."... Thus the theory of evolution explains the housekeeping relations of organisms mechanistically as the necessary consequences of effectual causes and so forms the monistic groundwork of ecology.


Opinions differ on who was the founder of modern ecological theory. Some mark Haeckel's definition as the beginning;[222] others say it was Eugenius Warming with the writing of Oecology of Plants: An Introduction to the Study of Plant Communities (1895),[223] or Carl Linnaeus' principles on the economy of nature that matured in the early 18th century.[224][225] Linnaeus founded an early branch of ecology that he called the economy of nature.[224] His works influenced Charles Darwin, who adopted Linnaeus' phrase on the economy or polity of nature in The Origin of Species.[220] Linnaeus was the first to frame the balance of nature as a testable hypothesis. Haeckel, who admired Darwin's work, defined ecology in reference to the economy of nature, which has led some to question whether ecology and the economy of nature are synonymous.[225]

From Aristotle until Darwin, the natural world was predominantly considered static and unchanging. Prior to The Origin of Species, there was little appreciation or understanding of the dynamic and reciprocal relations between organisms, their adaptations, and the environment.[212] An exception is the 1789 publication Natural History of Selborne by Gilbert White (1720–1793), considered by some to be one of the earliest texts on ecology.[226] While Charles Darwin is mainly noted for his treatise on evolution,[227] he was one of the founders of soil ecology,[228] and he made note of the first ecological experiment in The Origin of Species.[229] Evolutionary theory changed the way that researchers approached the ecological sciences.[230]
Nowhere can one see more clearly illustrated what may be called the sensibility of such an organic complex,--expressed by the fact that whatever affects any species belonging to it, must speedily have its influence of some sort upon the whole assemblage. He will thus be made to see the impossibility of studying any form completely, out of relation to the other forms,--the necessity for taking a comprehensive survey of the whole as a condition to a satisfactory understanding of any part.
Since 1900
Modern ecology is a young science that first attracted substantial scientific attention toward the end of the 19th century (around the same time that evolutionary studies were gaining scientific interest). Notable scientist Ellen Swallow Richards may have first introduced the term "oekology" (which eventually morphed into home economics) in the U.S. as early 1892.[232]
In the early 20th century, ecology transitioned from a more descriptive form of natural history to a more analytical form of scientific natural history.[216][219] Frederic Clements published the first American ecology book in 1905,[233] presenting the idea of plant communities as a superorganism. This publication launched a debate between ecological holism and individualism that lasted until the 1970s. Clements' superorganism concept proposed that ecosystems progress through regular and determined stages of seral development that are analogous to the developmental stages of an organism. The Clementsian paradigm was challenged by Henry Gleason,[234] who stated that ecological communities develop from the unique and coincidental association of individual organisms. This perceptual shift placed the focus back onto the life histories of individual organisms and how this relates to the development of community associations.[235]
The Clementsian superorganism theory was an overextended application of an idealistic form of holism.[31][104] The term "holism" was coined in 1926 by Jan Christiaan Smuts, a South African general and polarizing historical figure who was inspired by Clements' superorganism concept.[236][C] Around the same time, Charles Elton pioneered the concept of food chains in his classical book Animal Ecology.[80] Elton[80] defined ecological relations using concepts of food chains, food cycles, and food size, and described numerical relations among different functional groups and their relative abundance. Elton's 'food cycle' was replaced by 'food web' in a subsequent ecological text.[237] Alfred J. Lotka brought in many theoretical concepts applying thermodynamic principles to ecology.
In 1942, Raymond Lindeman wrote a landmark paper on the trophic dynamics of ecology, which was published posthumously after initially being rejected for its theoretical emphasis. Trophic dynamics became the foundation for much of the work to follow on energy and material flow through ecosystems. Robert E. MacArthur advanced mathematical theory, predictions and tests in ecology in the 1950s, which inspired a resurgent school of theoretical mathematical ecologists.[219][238][239] Ecology also has developed through contributions from other nations, including Russia's Vladimir Vernadsky and his founding of the biosphere concept in the 1920s[240] and Japan's Kinji Imanishi and his concepts of harmony in nature and habitat segregation in the 1950s.[241] Scientific recognition of contributions to ecology from non-English-speaking cultures is hampered by language and translation barriers.[240]
This whole chain of poisoning, then, seems to rest on a base of minute plants which must have been the original concentrators. But what of the opposite end of the food chain—the human being who, in probable ignorance of all this sequence of events, has rigged his fishing tackle, caught a string of fish from the waters of Clear Lake, and taken them home to fry for his supper?
Ecology surged in popular and scientific interest during the 1960–1970s environmental movement. There are strong historical and scientific ties between ecology, environmental management, and protection.[219] The historic emphasis and poetic naturalist writings for protection was on wild places, from notable ecologists in the history of conservation biology, such as Aldo Leopold and Arthur Tansley, were far removed from urban centres where the concentration of pollution and environmental degradation is located.[219][243] Palamar (2008)[243] notes an overshadowing by mainstream environmentalism of pioneering women in the early 1900s who fought for urban health ecology (then called euthenics)[232] and brought about changes in environmental legislation. Women such as Ellen Swallow Richards and Julia Lathrop, among others, were precursors to the more popularized environmental movements after the 1950s.
In 1962, marine biologist and ecologist Rachel Carson's book Silent Spring helped to mobilize the environmental movement by alerting the public to toxic pesticides, such as DDT, bioaccumulating in the environment. Carson used ecological science to link the release of environmental toxins to human and ecosystem health. Since then, ecologists have worked to bridge their understanding of the degradation of the planet's ecosystems with environmental politics, law, restoration, and natural resources management.[20][219][243][244]
See also
- Agroecology
- Chemical ecology
- Cultural ecology
- Dialectical naturalism
- Earth science
- Ecological death
- Ecological psychology
- Ecology movement
- Euthenics
- Industrial ecology
- Information ecology
- Landscape ecology
- Natural resource
- Political ecology
- Restoration ecology
- Spiritual ecology
- Sustainable development
- Lists
Notes
- ^ In Ernst Haeckel's (1866) footnote where the term ecology originates, he also gives attribute to Ancient Greek: χώρας khōrā “χωρα”, meaning "dwelling place, distributional area" - quoted from Stauffer (1957)
- ^ This is a copy of Haeckel's original definition (Original: Haeckel, E. (1866) Generelle Morphologie der Organismen. Allgemeine Grundzige der organischen Formen- Wissenschaft, mechanisch begriindet durch die von Charles Darwin reformirte Descendenz-Theorie. 2 vols. Reimer, Berlin.) translated and quoted from Stauffer (1957).
- ^ Foster & Clark (2008) note how Smut's holism contrasts starkly against his racial political views as the father of apartheid.
- ^ First introduced in MacArthur & Wilson's (1967) book of notable mention in the history and theoretical science of ecology, The Theory of Island Biogeography
- ^ Aristotle wrote about this concept in Metaphysics (Quoted from The Internet Classics Archive translation by W. D. Ross. Book VIII, Part 6): "To return to the difficulty which has been stated with respect both to definitions and to numbers, what is the cause of their unity? In the case of all things which have several parts and in which the totality is not, as it were, a mere heap, but the whole is something beside the parts, there is a cause; for even in bodies contact is the cause of unity in some cases, and in others viscosity or some other such quality."
References
- ↑ Stadler, B.; Michalzik, B.; Müller, T. (1998). "Linking aphid ecology with nutrient fluxes in a coniferous forest". Ecology 79 (5): 1514–1525. doi:10.1890/0012-9658(1998)079[1514:LAEWNF]2.0.CO;2. ISSN 0012-9658.
- ↑ Humphreys, N. J.; Douglas, A. E. (1997). "Partitioning of symbiotic bacteria between generations of an insect: a quantitative study of a Buchnera sp. in the pea aphid (Acyrthosiphon pisum) reared at different temperatures". Applied and Environmental Microbiology 63 (8): 3294–3296. PMC 1389233. PMID 16535678.
- ↑ Liere, Heidi; Jackson, Doug; Vandermeer, John; Wilby, Andrew (20 September 2012). "Ecological Complexity in a Coffee Agroecosystem: Spatial Heterogeneity, Population Persistence and Biological Control". PLoS ONE 7 (9): e45508. Bibcode:2012PLoSO...745508L. doi:10.1371/journal.pone.0045508. PMC 3447771. PMID 23029061.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 4.10 4.11 Odum, E. P.; Barrett, G. W. (2005). Fundamentals of Ecology. Brooks Cole. p. 598. ISBN 978-0-534-42066-6.
- ↑ 5.0 5.1 O'Neill, D. L.; Deangelis, D. L.; Waide, J. B.; Allen, T. F. H. (1986). A Hierarchical Concept of Ecosystems. Princeton University Press. p. 253. ISBN 0-691-08436-X.
- ↑ Nachtomy, Ohad; Shavit, Ayelet; Smith, Justin (2002). "Leibnizian organisms, nested individuals, and units of selection". Theory in Biosciences 121 (2): 205. doi:10.1007/s12064-002-0020-9.
- ↑ 7.0 7.1 Holling, C. S. (2004). "Understanding the complexity of economic, ecological, and social systems". Ecosystems 4 (5): 390–405. doi:10.1007/s10021-001-0101-5.
- ↑ Levin, S. A. (1999). Fragile Dominion: Complexity and the Commons. Reading, MA: Perseus Books. ISBN 978-0-7382-0319-5.
- ↑ Noss, R. F.; Carpenter, A. Y. (1994). Saving Nature's Legacy: Protecting and Restoring Biodiversity. Island Press. p. 443. ISBN 978-1-55963-248-5.
- ↑ Noss, R. F. (1990). "Indicators for monitoring biodiversity: A hierarchical approach". Conservation Biology 4 (4): 355–364. doi:10.1111/j.1523-1739.1990.tb00309.x. JSTOR 2385928.
- ↑ 11.0 11.1 Scholes, R. J.; Mace, G. M.; Turner, W.; Geller, G. N.; Jürgens, N.; Larigauderie, A.; Muchoney, D.; Walther, B. A.; Mooney, H. A. (2008). "Toward a global biodiversity observing system". Science 321 (5892): 1044–1045. doi:10.1126/science.1162055. PMID 18719268.
- ↑ 12.0 12.1 Cardinale, Bradley J.; Duffy, J. Emmett; Gonzalez, Andrew; Hooper, David U.; Perrings, Charles; Venail, Patrick; Narwani, Anita; Mace, Georgina M.; Tilman, David; Wardle, David A.; Kinzig, Ann P.; Daily, Gretchen C.; Loreau, Michel; Grace, James B.; Larigauderie, Anne; Srivastava, Diane S.; Naeem, Shahid (6 June 2012). "Biodiversity loss and its impact on humanity". Nature 486 (7401): 59–67. Bibcode:2012Natur.486...59C. doi:10.1038/nature11148. PMID 22678280.
- ↑ Wilson, E. O. (2000). "A global biodiversity map". Science 289 (5488): 2279. doi:10.1126/science.289.5488.2279 (inactive 2014-02-02). PMID 11041790.
- ↑ Purvis, A.; Hector, A. (2000). "Getting the measure of biodiversity". Nature 405 (6783): 212–218. doi:10.1038/35012221. PMID 10821281.
- ↑ Ostfeld, R. S. (2009). "Biodiversity loss and the rise of zoonotic pathogens". Clinical Microbiology and Infection 15 (s1): 40–43. doi:10.1111/j.1469-0691.2008.02691.x. PMID 19220353.
- ↑ Tierney; Faber-Langendoen, Don; Mitchell, Brian R.; Shriver, W. Gregory; Gibbs, James P. (2009). "Monitoring and evaluating the ecological integrity of forest ecosystems". Frontiers in Ecology and the Environment 7 (6): 308–316. doi:10.1890/070176. Unknown parameter
|DUPLICATE_first2=ignored (help) - ↑ Ceballos, G.; Ehrlich, P. R. (2002). "Mammal population losses and the extinction crisis". Science 296 (5569): 904–907. Bibcode:2002Sci...296..904C. doi:10.1126/science.1069349. PMID 11988573. Retrieved 2010-03-16.
- ↑ Palumbi; S. R.; Allan, J. David; Beck, Michael W.; Fautin, Daphne G.; Fogarty, Michael J.; Halpern, Benjamin S.; Incze, Lewis S.; Leong, Jo-Ann (2009). "Managing for ocean biodiversity to sustain marine ecosystem services". Frontiers in Ecology and the Environment 7 (4): 204–211. doi:10.1890/070135.
- ↑ Wilcove, D. S.; Wikelski, M. (2008). "Going, going, gone: Is animal migration disappearing". PLoS Biology 6 (7): e188. doi:10.1371/journal.pbio.0060188. PMC 2486312. PMID 18666834.
- ↑ 20.0 20.1 20.2 Hammond, H. (2009). Maintaining Whole Systems on the Earth's Crown: Ecosystem-based Conservation Planning for the Boreal Forest. Slocan Park, BC: Silva Forest Foundation. p. 380. ISBN 978-0-9734779-0-0.
- ↑ 21.0 21.1 21.2 Whittaker, R. H.; Levin, S. A.; Root, R. B. (1973). "Niche, habitat, and ecotope". The American Naturalist 107 (955): 321–338. doi:10.1086/282837.
- ↑ Beyer, Hawthorne, L.; Haydon, Daniel, T.; Morales, Juan M.; Frair, Jacqueline L.; Hebblewhite, Mark; Mitchell, Michael; Matthiopoulos, Jason (2010). "The interpretation of habitat preference metrics under use–availability designs". Philosophical Transactions of the Royal Society B 365 (1550): 2245–2254. doi:10.1098/rstb.2010.0083. PMC 2894962. PMID 20566501.
- ↑ Schoener, T. W. (1975). "Presence and absence of habitat shift in some widespread lizard species". Ecological Monographs 45 (3): 233–258. doi:10.2307/1942423. JSTOR 1942423.
- ↑ Vitt, L. J.; Caldwell, J. P.; Zani, P. A.; Titus, T. A. (1997). "The role of habitat shift in the evolution of lizard morphology: Evidence from tropical Tropidurus". Proceedings of the National Academy of Sciences U.S.A 94 (8): 3828–3832. Bibcode:1997PNAS...94.3828V. doi:10.1073/pnas.94.8.3828. PMC 20526. PMID 9108063.
- ↑ 25.0 25.1 25.2 Wiens, J. J.; Graham, C. H. (2005). "Niche conservatism: Integrating evolution, ecology, and conservation biology". Annual Review of Ecology, Evolution, and Systematics 36: 519–539. doi:10.1146/annurev.ecolsys.36.102803.095431.
- ↑ Hutchinson, G. E. (1957). A Treatise on Limnology. New York, NY: Wiley. p. 1015. ISBN 0-471-42572-9.
- ↑ 27.0 27.1 Hutchinson, G. E. (1957). "Concluding remarks". Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427. doi:10.1101/SQB.1957.022.01.039.
- ↑ 28.0 28.1 28.2 Begon, M.; Townsend, C. R.; Harper, J. L. (2005). Ecology: From Individuals to Ecosystems (4th ed.). Wiley-Blackwell. p. 752. ISBN 1-4051-1117-8.
- ↑ D. L., Hardesty (1975). "The niche concept: suggestions for its use in human ecology". Human Ecology 3 (2): 71–85. doi:10.1007/BF01552263. JSTOR 4602315.
- ↑ Pearman, P. B.; Guisan, A.; Broennimann, O.; Randin, C. F. (2008). "Niche dynamics in space and time". Trends in Ecology & Evolution 23 (3): 149–158. doi:10.1016/j.tree.2007.11.005. PMID 18289716.
- ↑ 31.0 31.1 31.2 31.3 31.4 Levins, R.; Lewontin, R. (1980). "Dialectics and reductionism in ecology". Synthese 43: 47–78.
- ↑ Hardin, G. (1960). "The competitive exclusion principal". Science 131 (3409): 1292–1297. Bibcode:1960Sci...131.1292H. doi:10.1126/science.131.3409.1292. PMID 14399717.
- ↑ Scheffer, M.; van Nes, E. H. (2006). "Self-organized similarity, the evolutionary emergence of groups of similar species". Proceedings of the National Academy of Sciences 103 (16): 6230–6235. Bibcode:2006PNAS..103.6230S. doi:10.1073/pnas.0508024103.
- ↑ Hastings, Alan; Byers, James E.; Crooks, Jeffrey A.; Cuddington, Kim; Jones, Clive G.; Lambrinos, John G.; Talley, Theresa S.; Wilson, William G. (2007). "Ecosystem engineering in space and time". Ecology Letters 10 (2): 153–164. doi:10.1111/j.1461-0248.2006.00997.x. PMID 17257103.
- ↑ Jones, Clive G.; Lawton, John H.; Shachak, Moshe (1994). "Organisms as ecosystem engineers". Oikos 69 (3): 373–386. doi:10.2307/3545850. JSTOR 3545850.
- ↑ 36.0 36.1 Laland, K. N.; Odling-Smee, F. J.; Feldman, M. W. (1999). "Evolutionary consequences of niche construction and their implications for ecology". Proceedings of the National Academy of Sciences of the United States of America 96 (18): 10242–10247. Bibcode:1999PNAS...9610242L. doi:10.1073/pnas.96.18.10242. PMC 17873. PMID 10468593.
- ↑ Wright, J. P.; Jones, C.G. (2006). "The concept of organisms as ecosystem engineers ten years on: Progress, limitations, and challenges". BioScience 56 (3): 203–209. doi:10.1641/0006-3568(2006)056[0203:TCOOAE]2.0.CO;2. ISSN 0006-3568.
- ↑ Hughes, D. P.; Pierce, N. E.; Boomsma, J. J. (2008). "Social insect symbionts: evolution in homeostatic fortresses". Trends in Ecology & Evolution 23 (12): 672–677. doi:10.1016/j.tree.2008.07.011. PMID 18951653.
- ↑ Palmer, M.; White, P. S. (1994). "On the existence of ecological communities". Journal of Vegetation Sciences 5 (2): 279–282. doi:10.2307/3236162. JSTOR 3236162.
- ↑ Prentice; I. C.; Harrison, S. P.; Leemans, R.; Monserud, R. A.; Solomon, A. M. (1992). "Special paper: A global biome model based on plant physiology and dominance, soil properties and climate". Journal of Biogeography 19 (2): 117–134. doi:10.2307/2845499. JSTOR 2845499.
- ↑ Turnbaugh, Peter J.; Ley, Ruth E.; Hamady, Micah; Fraser-Liggett, Claire M.; Knight, Rob; Gordon, Jeffrey I. (2007). "The human microbiome project". Nature 449 (7164): 804–810. Bibcode:2007Natur.449..804T. doi:10.1038/nature06244. PMID 17943116.
- ↑ DeLong, E. F. (2009). "The microbial ocean from genomes to biomes". Nature 459 (7244): 200–206. Bibcode:2009Natur.459..200D. doi:10.1038/nature08059. PMID 19444206.
- ↑ Igamberdiev, Abir U.; Lea, P. J. (2006). "Land plants equilibrate O2 and CO2 concentrations in the atmosphere". Photosynthesis Research 87 (2): 177–194. doi:10.1007/s11120-005-8388-2. PMID 16432665.
- ↑ Lovelock, J.; Margulis, Lynn (1973). "Atmospheric homeostasis by and for the biosphere: The Gaia hypothesis". Tellus 26: 2–10. Bibcode:1974Tell...26....2L. doi:10.1111/j.2153-3490.1974.tb01946.x.
- ↑ 45.0 45.1 45.2 Lovelock, J. (2003). "The living Earth". Nature 426 (6968): 769–770. Bibcode:2003Natur.426..769L. doi:10.1038/426769a. PMID 14685210.
- ↑ Waples, R. S.; Gaggiotti, O. (2006). "What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity". Molecular Ecology 15 (6): 1419–1439. doi:10.1111/j.1365-294X.2006.02890.x. PMID 16629801.
- ↑ 47.0 47.1 47.2 Turchin, P. (2001). "Does population ecology have general laws?". Oikos 94 (1): 17–26. doi:10.1034/j.1600-0706.2001.11310.x.
- ↑ 48.0 48.1 Vandermeer, J. H.; Goldberg, D. E. (2003). Population Ecology: First Principles. Woodstock, Oxfordshire: Princeton University Press. ISBN 0-691-11440-4.
- ↑ Berryman, A. A. (1992). "The origins and evolution of predator-prey theory". Ecology 73 (5): 1530–1535. doi:10.2307/1940005. JSTOR 1940005.
- ↑ Anderson, D. R.; Burnham, K. P.; Thompson, W. L. (2000). "Null hypotheses testing: Problems, prevalence, and an alternative". J. Wildl. Mngt. 64 (4): 912–923.
- ↑ Johnson, J. B.; Omland, K. S. (2004). "Model selection in ecology and evolution". Trends in Ecology and Evolution 19 (2): 101–108. doi:10.1016/j.tree.2003.10.013. PMID 16701236.
- ↑ Levins, R. (1969). "Some demographic and genetic consequences of environmental heterogeneity for biological control". Bulletin of the Entomological Society of America 15: 237–240. ISBN 978-0-231-12680-9.
- ↑ Levins, R. (1970). "Extinction". In Gerstenhaber, M. Some Mathematical Questions in Biology. pp. 77–107. ISBN 978-0-8218-1152-8.
- ↑ Smith, M. A.; Green, D. M. (2005). "Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations?". Ecography 28 (1): 110–128. doi:10.1111/j.0906-7590.2005.04042.x.
- ↑ Hanski, I. (1998). "Metapopulation dynamics". Nature 396 (6706): 41–49. Bibcode:1998Natur.396...41H. doi:10.1038/23876.
- ↑ Nebel, S. (2010). "Animal migration". Nature Education Knowledge 10 (1): 29.
- ↑ Clark, J. S.; Fastie, C.; Hurtt, G.; Jackson, S. T.; Johnson, C.; King, G. A. (1998). "Reid's paradox of rapid plant migration". BioScience 48 (1): 13–24. doi:10.2307/1313224.
- ↑ Dingle, H. (1996-01-18). Migration: The Biology of Life on the Move. Oxford University Press. p. 480. ISBN 0-19-509723-8.
- ↑ Hanski, I.; Gaggiotti, O. E., eds. (2004). Ecology, Genetics and Evolution of Metapopulations. Burlington, MA: Elsevier Academic Press. ISBN 0-12-323448-4.
- ↑ MacKenzie; D.I. (2006). Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence. London, UK: Elsevier Academic Press. p. 324. ISBN 978-0-12-088766-8.
- ↑ Johnson, M. T.; Strinchcombe, J. R. (2007). "An emerging synthesis between community ecology and evolutionary biology". Trends in Ecology and Evolution 22 (5): 250–257. doi:10.1016/j.tree.2007.01.014. PMID 17296244.
- ↑ Brinson, M. M.; Lugo, A. E.; Brown, S (1981). "Primary Productivity, Decomposition and Consumer Activity in Freshwater Wetlands". Annual Review of Ecology and Systematics 12: 123–161. doi:10.1146/annurev.es.12.110181.001011.
- ↑ 63.0 63.1 Davic, R. D.; Welsh, H. H. (2004). "On the ecological role of salamanders". Annual Review of Ecology and Systematics 35: 405–434. doi:10.1146/annurev.ecolsys.35.112202.130116.
- ↑ 64.0 64.1 Tansley, A. G. (1935). "The use and abuse of vegetational concepts and terms". Ecology 16 (3): 284–307. doi:10.2307/1930070. JSTOR 1930070.
- ↑ Marsh, G. P. (1864). Man and Nature: Physical Geography as Modified by Human Action. Cambridge, MA: Belknap Press. p. 560.
- ↑ O'Neil, R. V. (2001). "Is it time to bury the ecosystem concept? (With full military honors, of course!)". Ecology 82 (12): 3275–3284. doi:10.1890/0012-9658(2001)082[3275:IITTBT]2.0.CO;2. ISSN 0012-9658.
- ↑ Levin, S. A. (1998). "Ecosystems and the biosphere as complex adaptive systems". Ecosystems 1 (5): 431–436. doi:10.1007/s100219900037. CiteSeerX: 10.1.1.83.6318.
- ↑ Pimm, S. (2002). Food Webs. University of Chicago Press. p. 258. ISBN 978-0-226-66832-1.
- ↑ 69.0 69.1 Pimm, S. L.; Lawton, J. H.; Cohen, J. E. (1991). "Food web patterns and their consequences" (PDF). Nature 350 (6320): 669–674. Bibcode:1991Natur.350..669P. doi:10.1038/350669a0.
- ↑ Worm, B.; Duffy, J. E. (2003). "Biodiversity, productivity and stability in real food webs". Trends in Ecology and Evolution 18 (12): 628–632. doi:10.1016/j.tree.2003.09.003.
- ↑ McCann, K. (2007). "Protecting biostructure". Nature 446 (7131): 29. Bibcode:2007Natur.446...29M. doi:10.1038/446029a. PMID 17330028.
- ↑ Wilbur, H. W. (1997). "Experimental ecology of food webs: Complex systems in temporary ponds" (PDF). Ecology 78 (8): 2279–2302. doi:10.1890/0012-9658(1997)078[2279:EEOFWC]2.0.CO;2. ISSN 0012-9658.
- ↑ Emmerson, M.; Yearsley, J. M. (2004). "Weak interactions, omnivory and emergent food-web properties". Philosophical Transactions of the Royal Society B 271 (1537): 397–405. doi:10.1098/rspb.2003.2592.
- ↑ Krause, A. E.; Frank, K. A.; Mason, D. M.; Ulanowicz, R. E.; Taylor, W. W. (2003). "Compartments revealed in food-web structure". Nature 426 (6964): 282–285. Bibcode:2003Natur.426..282K. doi:10.1038/nature02115. PMID 14628050.
- ↑ Egerton, Frank N. (2007). "Understanding food chains and food webs, 1700–1970". Bulletin of the Ecological Society of America 88: 50–69. doi:10.1890/0012-9623(2007)88[50:UFCAFW]2.0.CO;2. ISSN 0012-9623.
- ↑ Shurin, J. B.; Gruner, D. S.; Hillebrand, H. (2006). "All wet or dried up? Real differences between aquatic and terrestrial food webs". Proceedings of the Royal Society B 273 (1582): 1–9. doi:10.1098/rspb.2005.3377. PMC 1560001. PMID 16519227.
- ↑ Edwards, J.; Fraser, K. (1983). "Concept maps as reflectors of conceptual understanding". Research in Science Education 13: 19–26. Bibcode:1983RScEd..13...19E. doi:10.1007/BF02356689.
- ↑ Hairston Jr., N. G.; Hairston Sr., N. G. (1993). "Cause-effect relationships in energy flow, trophic structure, and interspecific interactions". The American Naturalist 142 (3): 379–411. doi:10.1086/285546.
- ↑ Duffy; Cardinale, Bradley J.; France, Kristin E.; McIntyre, Peter B.; Thébault, Elisa; Loreau, Michel (2007). "The functional role of biodiversity in ecosystems: incorporating trophic complexity". Ecology Letters 10 (6): 522–538. doi:10.1111/j.1461-0248.2007.01037.x. PMID 17498151. Unknown parameter
|DUPLICATE_last2=ignored (help) - ↑ 80.0 80.1 80.2 Elton, C. S. (1927). Animal Ecology. London, UK.: Sidgwick and Jackson. ISBN 0-226-20639-4.
- ↑ Davic, R. D. (2003). "Linking keystone species and functional groups: a new operational definition of the keystone species concept". Conservation Ecology 7 (1): r11.
- ↑ Oksanen, L. (1991). "Trophic levels and trophic dynamics: A consensus emerging?". Trends in Ecology and Evolution 6 (2): 58–60. doi:10.1016/0169-5347(91)90124-G. PMID 21232425.
- ↑ Loehle, C.; Pechmann, Joseph H. K. (1988). "Evolution: The missing ingredient in systems ecology". The American Naturalist 132 (9): 884–899. doi:10.1086/284895. JSTOR 2462267.
- ↑ Ulanowicz, R. E.; Kemp, W. Michael (1979). "Toward canonical trophic aggregations". The American Naturalist 114 (6): 871–883. doi:10.1086/283534. JSTOR 2460557.
- ↑ Li, B. (2000). "Why is the holistic approach becoming so important in landscape ecology?". Landscape and Urban Planning 50 (1–3): 27–41. doi:10.1016/S0169-2046(00)00078-5.
- ↑ Polis, G. A.; Strong, D. R. (1996). "Food web complexity and community dynamics". The American Naturalist 147 (5): 813–846. doi:10.1086/285880.
- ↑ Thompson, R. M.; Hemberg, M.; Starzomski, B. M.; Shurin, J. B. (2007). "Trophic levels and trophic tangles: The prevalence of omnivory in real food webs". Ecology 88 (3): 612–617. doi:10.1890/05-1454. PMID 17503589.
- ↑ Fischer, J.; Lindenmayer, D. B.; Manning, A. D. (2006). "Biodiversity, ecosystem function, and resilience: ten guiding principles for commodity production landscapes". Frontiers in Ecology and the Environment 4 (2): 80–86. doi:10.1890/1540-9295(2006)004[0080:BEFART]2.0.CO;2. ISSN 1540-9295.
- ↑ Libralato, S.; Christensen, V.; Pauly, D. (2006). "A method for identifying keystone species in food web models". Ecological Modelling 195 (3–4): 153–171. doi:10.1016/j.ecolmodel.2005.11.029.
- ↑ 90.0 90.1 Mills, L. S.; Soule, M. E.; Doak, D. F. (1993). "The keystone-species concept in ecology and conservation". BioScience 43 (4): 219–224. doi:10.2307/1312122. JSTOR 1312122.
- ↑ Anderson, P. K. (1995). "Competition, predation, and the evolution and extinction of Stellar's sea cow, Hydrodamalis gigas". Marine Mammal Science 11 (3): 391–394. doi:10.1111/j.1748-7692.1995.tb00294.x.
- ↑ Polis, G. A.; Sears, Anna L. W.; Huxel, Gary R.; Strong, Donald R.; Maron, John (2000). "When is a trophic cascade a trophic cascade?". Trends in Ecology and Evolution 15 (11): 473–475. doi:10.1016/S0169-5347(00)01971-6. PMID 11050351.
- ↑ Novikoff, AB (1945). "The concept of integrative levels and biology". Science 101 (2618): 209–215. Bibcode:1945Sci...101..209N. doi:10.1126/science.101.2618.209. PMID 17814095.
- ↑ Schneider, D. D. (2001). "The rise of the concept of scale in ecology". BioScience 51 (7): 545–553. doi:10.1641/0006-3568(2001)051[0545:TROTCO]2.0.CO;2. ISSN 0006-3568.
- ↑ Molnar, J.; Marvier, M.; Kareiva, P. (2004). "The sum is greater than the parts". Conservation Biology 18 (6): 1670–1671. doi:10.1111/j.1523-1739.2004.00l07.x.
- ↑ Loehle, C. (2004). "Challenges of ecological complexity". Ecological Complexity 1 (1): 3–6. doi:10.1016/j.ecocom.2003.09.001.
- ↑ 97.0 97.1 Odum, E. P. (1977). "The emergence of ecology as a new integrative discipline". Science 195 (4284): 1289–1293. Bibcode:1977Sci...195.1289O. doi:10.1126/science.195.4284.1289. PMID 17738398.
- ↑ Scheffer, M.; Carpenter, S.; Foley, J. A.; Walker, B.; Walker, B. (2001). "Catastrophic shifts in ecosystems". Nature 413 (6856): 591–596. Bibcode:2001Natur.413..591S. doi:10.1038/35098000. PMID 11595939.
- ↑ "Welcome to ILTER — ILTER". International Long Term Ecological Research. Retrieved 2010-03-16.
- ↑ Silverton, Jonathan; Poulton, Paul; Johnston, Edward; Edwards, Grant; Heard, Matthew; Biss, Pamela M. (2006). "The Park Grass Experiment 1856–2006: Its contribution to ecology". Journal of Ecology 94 (4): 801–814. doi:10.1111/j.1365-2745.2006.01145.x.
- ↑ "Hubbard Brook Ecosystem Study Front Page". Retrieved 2010-03-16.
- ↑ 102.0 102.1 102.2 102.3 Liu, J.; Dietz, Thomas; Carpenter, Stephen R.; Folke, Carl; Alberti, Marina; Redman, Charles L.; Schneider, Stephen H.; Ostrom, Elinor; Pell, Alice N. (2009). "Coupled human and natural systems". AMBIO: A Journal of the Human Environment 36 (8): 639–649. doi:10.1579/0044-7447(2007)36[639:CHANS]2.0.CO;2. ISSN 0044-7447.
- ↑ Mikkelson, G. M. (2010). "Part-whole relationships and the unity of ecology". In Skipper, R. A.; Allen, C.; Ankeny, R.; Craver, C. F.; Darden, L.; Richardson, R.C. Philosophy Across the Life Sciences. Cambridge, MA: MIT Press.
- ↑ 104.0 104.1 Wilson, D. S. (1988). "Holism and reductionism in evolutionary ecology". Oikos 53 (2): 269–273. doi:10.2307/3566073. JSTOR 3566073.
- ↑ Miles, D. B.; Dunham, A. E. (1993). "Historical perspectives in ecology and evolutionary biology: The use of phylogenetic comparative analyses". Annual Review of Ecology and Systematics 24: 587–619. doi:10.1146/annurev.es.24.110193.003103.
- ↑ Craze, P., ed. (August 2, 2012). "Trends in Ecology and Evolution". Cell Press, Elsevier, Inc.
- ↑ 107.0 107.1 107.2 107.3 107.4 107.5 107.6 107.7 107.8 Allee, W. C.; Park, O.; Emerson, A. E.; Park, T.; Schmidt, K. P. (1949). Principles of Animal Ecology. W. B. Sunders, Co. p. 837. ISBN 0-7216-1120-6.
- ↑ 108.0 108.1 108.2 108.3 108.4 Rickleffs, Robert, E. (1996). The Economy of Nature. University of Chicago Press. p. 678. ISBN 0-7167-3847-3.
- ↑ Yoshida, T. "Rapid evolution drives ecological dynamics in a predator–prey system". Nature Publishing Group.
- ↑ Karban, R. (2008). "Plant behaviour and communication". Ecology Letters 11 (7): 727–739. doi:10.1111/j.1461-0248.2008.01183.x. PMID 18400016.
- ↑ Tinbergen, N. (1963). "On aims and methods of ethology" (PDF). Zeitschrift für Tierpsychologie 20 (4): 410–433. doi:10.1111/j.1439-0310.1963.tb01161.x.
- ↑ Hamner, W. M. (1985). "The importance of ethology for investigations of marine zooplankton". Bulletin of Marine Science 37 (2): 414–424.
- ↑ 113.0 113.1 Strassmann, J. E.; Zhu, Y.; Queller, D. C. (2000). "Altruism and social cheating in the social amoeba Dictyostelium discoideum". Nature 408 (6815): 965–967. doi:10.1038/35050087. PMID 11140681.
- ↑ Sakurai, K. (1985). "An attelabid weevil (Euops splendida) cultivates fungi". Journal of Ethology 3 (2): 151–156. doi:10.1007/BF02350306.
- ↑ Anderson, J. D. (1961). "The courtship behaviour of Ambystoma macrodactylum croceum". Copeia 2 (2): 132–139. JSTOR 1439987.
- ↑ "Behavioral Ecology". International Society for Behavioral Ecology. Retrieved 15 April 2011.
- ↑ Gould, Stephen J.; Vrba, Elizabeth S. (1982). "Exaptation–a missing term in the science of form". Paleobiology 8 (1): 4–15.
- ↑ 118.0 118.1 118.2 118.3 Wilson, E. O. (2000). Sociobiology: The New Synthesis (25th anniversary ed.). President and Fellows of Harvard College. ISBN 978-0-674-00089-6.
- ↑ Ives, A. R.; Cardinale, B. J.; Snyder, W. E. (2004). "A synthesis of subdisciplines: Predator–prey interactions, and biodiversity and ecosystem functioning". Ecology Letters 8 (1): 102–116. doi:10.1111/j.1461-0248.2004.00698.x.
- ↑ Krebs, J. R.; Davies, N. B. (1993). An Introduction to Behavioural Ecology. Wiley-Blackwell. p. 432. ISBN 978-0-632-03546-5.
- ↑ Webb, J. K.; Pike, D. A.; Shine, R. (2010). "Olfactory recognition of predators by nocturnal lizards: safety outweighs thermal benefits". Behavioural Ecology 21 (1): 72–77. doi:10.1093/beheco/arp152.
- ↑ Cooper, W. E.; Frederick, W. G. (2010). "Predator lethality, optimal escape behavior, and autotomy". Behavioral Ecology 21 (1): 91–96. doi:10.1093/beheco/arp151.
- ↑ Kodric-Brown, A.; Brown, J. H. (1984). "Truth in advertising: The kinds of traits favored by sexual selection". The American Naturalist 124 (3): 309–323. doi:10.1086/284275.
- ↑ Sherman, P. W.; Lacey, E. A.; Reeve, H. K.; Keller, L. (1995). "The eusociality continuum" (PDF). Behavioural Ecology 6 (1): 102–108.
- ↑ Wilson, D. S.; Wilson, E. O. (2007). "Rethinking the theoretical foundation of sociobiology". The Quarterly Review of Biology 82 (4): 327–348. doi:10.1086/522809. PMID 18217526.
- ↑ Page, R. D. M. (1991). "Clocks, clades, and cospeciation: Comparing rates of evolution and timing of cospeciation events in host-parasite assemblages". Systematic Zoology 40 (2): 188–198. doi:10.2307/2992256. JSTOR 2992256.
- ↑ Herre, E. A.; Knowlton, N.; Mueller, U. G.; Rehner, S. A. (1999). "The evolution of mutualisms: exploring the paths between conflict and cooperation". Trends in Ecology and Evolution 14 (2): 49–53. doi:10.1016/S0169-5347(98)01529-8. PMID 10234251.
- ↑ Gilbert, F. S. (1990). Insect life cycles: Genetics, evolution, and co-ordination. New York, NY: Springer-Verlag. p. 258. ISBN 0-387-19550-5.
- ↑ Kiers, E. T.; van der Heijden, M. G. A. (2006). "Mutualistic stability in the arbuscular mycorrhizal symbiosis: Exploring hypotheses of evolutionary cooperation". Ecology 87 (7): 1627–1636. doi:10.1890/0012-9658(2006)87[1627:MSITAM]2.0.CO;2. ISSN 0012-9658. PMID 16922314.
- ↑ Strain, B. R. (1985). "Physiological and ecological controls on carbon sequestering in terrestrial ecosystems". Biogeochemistry 1 (3): 219–232. doi:10.1007/BF02187200.
- ↑ Bronstein, J. L. (2001). "The exploitation of mutualisms". Ecology Letters 4 (3): 277–287. doi:10.1046/j.1461-0248.2001.00218.x.
- ↑ Irwin, Rebecca E.; Manson, Judith L.; Jessamyn S.; Richardson, Leif (2010). "Nectar robbing: Ecological and evolutionary perspectives". Annual Review of Ecology, Evolution, and Systematics 41 (2): 271–292. doi:10.1146/annurev.ecolsys.110308.120330. Unknown parameter
|DUPLICATE_last2=ignored (help) - ↑ Boucher, D. H.; James, S.; Keeler, K. H. (1982). "The ecology of mutualism". Annual Review of Ecology and Systematics 13: 315–347. doi:10.1146/annurev.es.13.110182.001531.
- ↑ King, K. C.; Delph, L. F.; Jokela, J.; Lively, C. M. (2009). "The geographic mosaic of sex and the Red Queen". Current Biology 19 (17): 1438–1441. doi:10.1016/j.cub.2009.06.062. PMID 19631541.
- ↑ 135.0 135.1 Parenti, L. R.; Ebach, M. C. (2009). Comparative Biogeography: Discovering and Classifying Biogeographical Patterns of a Dynamic Earth. London, England: University of California Press. ISBN 978-0-520-25945-4.
- ↑ "Journal of Biogeography". Wiley. Retrieved August 3, 2012.
- ↑ 137.0 137.1 MacArthur, R.; Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton, NJ: Princeton University Press.
- ↑ 138.0 138.1 Wiens, J. J.; Donoghue, M. J. (2004). "Historical biogeography, ecology and species richness". Trends in Ecology and Evolution 19 (12): 639–644. doi:10.1016/j.tree.2004.09.011. PMID 16701326.
- ↑ Morrone, J. J.; Crisci, J. V. (1995). "Historical biogeography: Introduction to methods". Annual Review of Ecology and Systematics 26: 373–401. doi:10.1146/annurev.es.26.110195.002105.
- ↑ Svenning, Jens-Christian; Condi, R. (2008). "Biodiversity in a warmer world". Science 322 (5899): 206–207. doi:10.1126/science.1164542. PMID 18845738.
- ↑ Landhäusser, Simon M.; Deshaies, D.; Lieffers, V. J. (2009). "Disturbance facilitates rapid range expansion of aspen into higher elevations of the Rocky Mountains under a warming climate". Journal of Biogeography 37 (1): 68–76. doi:10.1111/j.1365-2699.2009.02182.x.
- ↑ Reznick, D.; Bryant, M. J.; Bashey, F. (2002). "r- and K-selection revisited: The role of population regulation in life-history evolution". Ecology 83 (6): 1509–1520. doi:10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2. ISSN 0012-9658.
- ↑ Pianka, E. R. (1972). "r and K Selection or b and d Selection?". The American Naturalist 106 (951): 581–588. doi:10.1086/282798.
- ↑ Rieseberg, L. (ed.). "Molecular Ecology". Molecular Ecology (Wiley). doi:10.1111/(ISSN)1365-294X.
- ↑ 145.0 145.1 Avise, J. (1994). Molecular Markers, Natural History and Evolution. Kluwer Academic Publishers. ISBN 0-412-03771-8.
- ↑ O'Brian, E.; Dawson, R. (2007). "Context-dependent genetic benefits of extra-pair mate choice in a socially monogamous passerine". Behavioral Ecology and Sociobiology 61 (5): 775–782. doi:10.1007/s00265-006-0308-8.
- ↑ Avise, J. (2000). Phylogeography: The History and Formation of Species. President and Fellows of Harvard College. ISBN 0-674-66638-0.
- ↑ Rachel Carson (1962). ""Silent Spring" (excerpt)". Houghton Miffin. Retrieved 4 October 2012.
- ↑ 149.0 149.1 Young, G. L. (1974). "Human ecology as an interdisciplinary concept: A critical inquiry". Advances in Ecological Research. Advances in Ecological Research 8: 1–105. doi:10.1016/S0065-2504(08)60277-9. ISBN 978-0-12-013908-8.
- ↑ Gross, M. (2004). "Human geography and ecological sociology: the unfolding of human ecology, 1890 to 1930 – and beyond". Social Science History 28 (4): 575–605. doi:10.1215/01455532-28-4-575.
- ↑ "Millennium Ecosystem Assessment – Synthesis Report". United Nations. 2005. Retrieved 4 February 2010.
- ↑ de Groot, R. S.; Wilson, M. A.; Boumans, R. M. J. (2002). "A typology for the classification, description and valuation of ecosystem functions, goods and services". Ecological Economics 41 (3): 393–408. doi:10.1016/S0921-8009(02)00089-7.
- ↑ Aguirre, A. A. (2009). "Biodiversity and human health". EcoHealth 6: 153–156. doi:10.1007/s10393-009-0242-0.
- ↑ 154.0 154.1 154.2 Grumbine, R. E. (1994). "What is ecosystem management?". Conservation Biology 8 (1): 27–38.
- ↑ Wilson, E. O. (1992). The Diversity of Life. Harvard University Press. p. 440. ISBN 978-0-674-05817-0.
- ↑ Slocombe, D. S. (1993). Implementing ecosystem-based management 43 (9). pp. 612–622. JSTOR 1312148.
- ↑ Hobss, R. J.; Harris, J. A. (2001). "Restoration ecology: Repairing the Earth's ecosystems in the new millennium". Restoration Ecology 9 (2): 239–246. doi:10.1046/j.1526-100x.2001.009002239.x.
- ↑ Mason, H. L.; Langenheim, J. H. (1957). "Language analysis and the concept "environment"". Ecology 38 (2): 325–340. doi:10.2307/1931693. JSTOR 1931693.
- ↑ Kleese, D. A. (2001). "Nature and nature in Psychology". Journal of Theoretical and Philosophical Psychology 21: 61–79. doi:10.1037/h0091199.
- ↑ Campbell, Neil A.; Williamson, Brad; Heyden, Robin J. (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 0-13-250882-6.
- ↑ 161.0 161.1 161.2 161.3 161.4 Kormondy, E. E. (1995). Concepts of Ecology (4th ed.). Benjamin Cummings. ISBN 0-13-478116-3.
- ↑ 162.0 162.1 Hughes, A. R. "Disturbance and diversity: an ecological chicken and egg problem". Nature Education Knowledge 1 (8): 26.
- ↑ Levin, S. A. (1992). "The problem of pattern and scale in ecology: The Robert H. MacArthur Award". Ecology 73 (6): 1943–1967. doi:10.2307/1941447. JSTOR 1941447. Retrieved 2010-03-16.
- ↑ 164.0 164.1 Holling, C. S. (1973). "Resilience and stability of ecological systems". Annual Review of Ecology and Systematics 4 (1): 1–23. doi:10.1146/annurev.es.04.110173.000245. JSTOR 2096802.
- ↑ 165.0 165.1 Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. (2004). "Regime shifts, resilience, and biodiversity in ecosystem management". Annual Review of Ecology and Systematics 35: 557–581. doi:10.1146/annurev.ecolsys.35.021103.105711. JSTOR 2096802.
- ↑ Morgan Ernest, S. K.; Enquist, Brian J.; Brown, James H.; Charnov, Eric L.; Gillooly, James F.; Savage, Van M.; White, Ethan P.; Smith, Felisa A.; Hadly, Elizabeth A.; Haskell, John P.; Lyons, S. Kathleen; Maurer, Brian A.; Niklas, Karl J.; Tiffney, Bruce (2003). "Thermodynamic and metabolic effects on the scaling of production and population energy use". Ecology Letters 6 (11): 990–995. doi:10.1046/j.1461-0248.2003.00526.x.
- ↑ Allègre, Claude J.; Manhès, Gérard; Göpel, Christa (1995). "The age of the Earth". Geochimica et Cosmochimica Acta 59 (8): 1455–1456. Bibcode:1995GeCoA..59.1445A. doi:10.1016/0016-7037(95)00054-4.
- ↑ Wills, C.; Bada, J. (2001). The Spark of Life: Darwin and the Primeval Soup. Cambridge, MA: Perseus Publishing. ISBN 978-0-7382-0493-2.
- ↑ 169.0 169.1 Goldblatt, Colin; Lenton, Timothy M.; Watson, Andrew J. (2006). "Bistability of atmospheric oxygen and the Great Oxidation". Nature 443 (7112): 683–686. Bibcode:2006Natur.443..683G. doi:10.1038/nature05169. PMID 17036001.
- ↑ Catling, D. C.; Claire, M. W. (2005). "How Earth's atmosphere evolved to an oxic state: A status report". Earth and Planetary Science Letters 237: 1–20. Bibcode:2005E&PSL.237....1C. doi:10.1016/j.epsl.2005.06.013.
- ↑ 171.0 171.1 171.2 171.3 Cronk, J. K.; Fennessy, M. S. (2001). Wetland Plants: Biology and Ecology. Washington, D.C.: Lewis Publishers. ISBN 1-56670-372-7.
- ↑ Evans, D. H.; Piermarini, P. M.; Potts, W. T. W. (1999). "Ionic transport in the fish gill epithelium". Journal of Experimental Zoology 283 (7): 641–652. doi:10.1002/(SICI)1097-010X(19990601)283:7<641::AID-JEZ3>3.0.CO;2-W.
- ↑ Swenson, N. G.; Enquist, B. J. (2008). "The relationship between stem and branch wood specific gravity and the ability of each measure to predict leaf area". American Journal of Botany 95 (4): 516–519. doi:10.3732/ajb.95.4.516. PMID 21632377.
- ↑ Gabriel E. A.; Hicks, James W.; Manzani, Paulo R.; Andrade, Denis V.; Abe, Augusto S.; Wang, Tobias; Secor, Stephen M.; Garland Jr., Theodore (2010). "Phylogeny, ecology, and heart position in snakes". Physiological and Biochemical Zoology 83 (1): 43–54. doi:10.1086/648509. PMID 19968564. Unknown parameter
|DUPLICATE_last1=ignored (help) - ↑ Jacobsen, D. (2008). "Low oxygen pressure as a driving factor for the altitudinal decline in taxon richness of stream macroinvertebrates". Oecologia 154 (4): 795–807. doi:10.1007/s00442-007-0877-x. PMID 17960424.
- ↑ Wheeler, T. D.; Stroock, A. D. (2008). "The transpiration of water at negative pressures in a synthetic tree". Nature 455 (7210): 208–212. Bibcode:2008Natur.455..208W. doi:10.1038/nature07226. PMID 18784721.
- ↑ Pockman, W. T.; Sperry, J. S.; O'Leary, J. W. (1995). "Sustained and significant negative water pressure in xylem". Nature 378 (6558): 715–716. Bibcode:1995Natur.378..715P. doi:10.1038/378715a0.
- ↑ Zimmermann, U.; Schneider, H.; Wegner, L. H.; Wagner, M.; Szimtenings, A.; Haase, F.; Bentrup, F. W. (2002). "What are the driving forces for water lifting in the xylem conduit?". Physiologia Plantarum 114 (3): 327–335. doi:10.1034/j.1399-3054.2002.1140301.x. PMID 12060254.
- ↑ Kastak, D.; Schusterman, R. J. (1998). "Low-frequency amphibious hearing in pinnipeds: Methods, measurements, noise, and ecology". Journal of the Acoustical Society of America 103 (4): 2216–2228. Bibcode:1998ASAJ..103.2216K. doi:10.1121/1.421367. PMID 9566340.
- ↑ Nishiguchi, Y.; Ito, I.; Okada, M. (2010). "Structure and function of lactate dehydrogenase from hagfish". Marine Drugs 8 (3): 594–607. doi:10.3390/md8030594. PMC 2857353. PMID 20411117.
- ↑ Shimeta, J.; Jumars, P. A.; Lessard, E. J. (1995). "Influences of turbulence on suspension feeding by planktonic protozoa; experiments in laminar shear fields". Limnolology and Oceanography 40 (5): 845–859. doi:10.4319/lo.1995.40.5.0845.
- ↑ Etemad-Shahidi, A.; Imberger, J. (2001). "Anatomy of turbulence in thermally stratified lakes". Limnolology and Oceanography 46 (5): 1158–1170. doi:10.4319/lo.2001.46.5.1158.
- ↑ Wolf, B. O.; Walsberg, G. E. (2006). "Thermal effects of radiation and wind on a small bird and implications for microsite selection". Ecology 77 (7): 2228–2236. doi:10.2307/2265716. JSTOR 2265716.
- ↑ Daubenmire, R. (1975). "Floristic plant geography of eastern Washington and northern Idaho". Journal of Biogeography 2 (1): 1–18. doi:10.2307/3038197. JSTOR 3038197.
- ↑ Steele, C. A.; Carstens, B. C.; Storfer, A.; Sullivan, J. (2005). "Testing hypotheses of speciation timing in Dicamptodon copei and Dicamptodon aterrimus (Caudata: Dicamptodontidae)". Molecular Phylogenetics and Evolution 36 (1): 90–100. doi:10.1016/j.ympev.2004.12.001. PMID 15904859.
- ↑ Lenton, T. M.; Watson, A. (2000). "Redfield revisited. 2. What regulates the oxygen content of the atmosphere". Global Biogeochemical Cycles 14 (1): 249–268. Bibcode:2000GBioC..14..249L. doi:10.1029/1999GB900076.
- ↑ Lobert, J. M.; Warnatz, J. (1993). "Emissions from the combustion process in vegetation". In Crutzen, P. J.; Goldammer, J. G. Fire in the Environment: The Ecological, Atmospheric and Climatic Importance of Vegetation Fires. Wiley. ISBN 978-0-471-93604-6.
- ↑ Garren, K. H. (1943). "Effects of fire on vegetation of the southeastern United States". Botanical Review 9 (9): 617–654. doi:10.1007/BF02872506.
- ↑ Cooper, C. F. (1960). "Changes in vegetation, structure, and growth of southwestern pine forests since white settlement". Ecological Monographs 30 (2): 130–164. JSTOR 1948549.
- ↑ Cooper, C. F. (1961). "The ecology of fire". Scientific American 204 (4): 150–160. doi:10.1038/scientificamerican0461-150.
- ↑ van Wagtendonk, Jan W. (2007). "History and evolution of wildland fire use" (PDF). Fire Ecology Special Issue 3 (2): 3–17.
- ↑ Boerner, R. E. J. (1982). "Fire and nutrient cycling in temperate ecosystems". BioScience 32 (3): 187–192. doi:10.2307/1308941. JSTOR 1308941.
- ↑ Goubitz, S.; Werger, M. J. A.; Ne'eman, G. (2009). "Germination response to fire-related factors of seeds from non-serotinous and serotinous cones". Plant Ecology 169 (2): 195–204. doi:10.1023/A:1026036332277.
- ↑ Ne'eman, G.; Goubitz, S.; Nathan, R. (2004). "Reproductive traits of Pinus halepensis in the light of fire: a critical review". Plant Ecology 171 (1/2): 69–79. doi:10.1023/B:VEGE.0000029380.04821.99.
- ↑ Flematti, Gavin R.; Ghisalberti, Emilio L.; Dixon, Kingsley W.; Trengove, R. D. (2004). "A compound from smoke that promotes seed germination" (PDF). Science 305 (5686): 977. doi:10.1126/science.1099944. PMID 15247439.
- ↑ Coleman, D. C.; Corssley, D. A.; Hendrix, P. F. (2004). Fundamentals of Soil Ecology (2nd ed.). Academic Press. ISBN 0-12-179726-0.
- ↑ 197.0 197.1 Wilkinson, M. T.; Richards, P. J.; Humphreys, G. S. (2009). "Breaking ground: Pedological, geological, and ecological implications of soil bioturbation" (PDF). Earth-Science Reviews 97 (1–4): 257–272. doi:10.1016/j.earscirev.2009.09.005.
- ↑ Phillips, J. D. (2009). "Soils as extended composite phenotypes". Geoderma 149 (1–2): 143–151. doi:10.1016/j.geoderma.2008.11.028.
- ↑ Reinhardt, L.; Jerolmack, D.; Cardinale, B. J.; Vanacker, V.; Wright, J. "Dynamic interactions of life and its landscape: Feedbacks at the interface of geomorphology and ecology". Earth Surf. Process. Landforms 35: 78–101. doi:10.1002/esp (inactive 2014-02-02).
- ↑ Hasiotis, S. T. (2003). "Complex ichnofossils of solitary and social soil organisms: Understanding their evolution and roles in terrestrial paleoecosystems". Palaeogeography, Palaeoclimatology, Palaeoecology 192 (2): 259–320. doi:10.1016/S0031-0182(02)00689-2.
- ↑ Falkowski, P. G.; Fenchel, T.; Delong, E. F. (2008). "The microbial engines that drive Earth's biogeochemical cycles". Science 320 (5879): 1034–1039. Bibcode:2008Sci...320.1034F. doi:10.1126/science.1153213. PMID 18497287.
- ↑ Grace, J. (2004). "Understanding and managing the global carbon cycle". Journal of Ecology 92 (2): 189–202. doi:10.1111/j.0022-0477.2004.00874.x.
- ↑ Pearson, P. N.; Palmer, M. R. (2000). "Atmospheric carbon dioxide concentrations over the past 60 million years". Nature 406 (6797): 695–699. doi:10.1038/35021000. PMID 10963587.
- ↑ Pagani, M.; Zachos, J. C.; Freeman, K. H.; Tipple, B.; Bohaty, S. (2005). "Marked decline in atmospheric carbon dioxide concentrations during the Paleogene". Science 309 (5734): 600–603. Bibcode:2005Sci...309..600P. doi:10.1126/science.1110063. PMID 15961630.
- ↑ Zhuan, Q.; Melillo, J. M.; McGuire, A. D.; Kicklighter, D. W.; Prinn, R. G.; Steudler, P. A.; Felzer, B. S.; Hu, S. (2007). "Net emission of CH4 and CO2 in Alaska: Implications for the region's greenhouse gas budget". Ecological Applications 17 (1): 203–212. doi:10.1890/1051-0761(2007)017[0203:NEOCAC]2.0.CO;2. ISSN 1051-0761. PMID 17479846.
- ↑ Cox; Betts, Richard A.; Jones, Chris D.; Spall, Steven A.; Totterdell, Ian J. (2000). "Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model". Nature 408 (6809): 184–187. doi:10.1038/35041539. PMID 11089968. Unknown parameter
|DUPLICATE_last2=ignored (help) - ↑ Erwin, D. H. (2009). "Climate as a driver of evolutionary change". Current Biology 19 (14): R575–R583. doi:10.1016/j.cub.2009.05.047. PMID 19640496.
- ↑ Bamber, J. (2012). "Shrinking glaciers under scrutiny". Nature 482 (7386): 482–483. Bibcode:2012Natur.482..482B. doi:10.1038/nature10948. PMID 22318516.
- ↑ Heimann, Martin; Reichstein, Markus (2008). "Terrestrial ecosystem carbon dynamics and climate feedbacks". Nature 451 (7176): 289–292. Bibcode:2008Natur.451..289H. doi:10.1038/nature06591. PMID 18202646.
- ↑ Davidson, Eric A.; Janssens, Ivan A. (2006). "Temperature sensitivity of soil carbon decomposition and feedbacks to climate change". Nature 440 (7081): 165–173. Bibcode:2006Natur.440..165D. doi:10.1038/nature04514. PMID 16525463.
- ↑ 211.0 211.1 Egerton, F. N. (2001). "A history of the ecological sciences: early Greek origins" (PDF). Bulletin of the Ecological Society of America 82 (1): 93–97.
- ↑ 212.0 212.1 Benson, Keith R. (2000). "The emergence of ecology from natural history". Endeavour 24 (2): 59–62. doi:10.1016/S0160-9327(99)01260-0. PMID 10969480.
- ↑ Sober, E. "Evolution, population thinking, and essentialism". Philosophy of Science 47 (3): 350–383. JSTOR 186950.
- ↑ Hughes, J. D. (1985). "Theophrastus as ecologist". Environmental Review 9 (4): 296–306. doi:10.2307/3984460. JSTOR 3984460.
- ↑ Hughes, J. D. (1975). "Ecology in ancient Greece". Inquiry 18 (2): 115–125. doi:10.1080/00201747508601756.
- ↑ 216.0 216.1 Kingsland, S. (2004). "Conveying the intellectual challenge of ecology: An historical perspective". Frontiers in Ecology and the Environment 2 (7): 367–374. doi:10.1890/1540-9295(2004)002[0367:CTICOE]2.0.CO;2. ISSN 1540-9295.
- ↑ Rosenzweig, M. L. (2003). "Reconciliation ecology and the future of species diversity" (PDF). Oryx 37 (2): 194–205.
- ↑ Hawkins, B. A. (2001). "Ecology's oldest pattern". Endeavor 25 (3): 133. doi:10.1016/S0160-9327(00)01369-7.
- ↑ 219.0 219.1 219.2 219.3 219.4 219.5 McIntosh, R. P. (1985). The Background of Ecology: Concept and Theory. Cambridge University Press. p. 400. ISBN 0-521-27087-1.
- ↑ 220.0 220.1 220.2 Stauffer, R. C. (1957). "Haeckel, Darwin and ecology". The Quarterly Review of Biology 32 (2): 138–144. doi:10.1086/401754.
- ↑ Friederichs, K. (1958). "A definition of ecology and some thoughts about basic concepts". Ecology 39 (1): 154–159. doi:10.2307/1929981. JSTOR 1929981.
- ↑ Hinchman, L. P.; Hinchman, S. K. (2007). "What we owe the Romantics". Environmental Values 16 (3): 333–354. doi:10.3197/096327107X228382.
- ↑ Goodland, R. J. (1975). "The tropical origin of ecology: Eugen Warming's jubilee". Oikos 26 (2): 240–245. doi:10.2307/3543715. JSTOR 3543715.
- ↑ 224.0 224.1 Egerton, F. N. (2007). "A history of the ecological sciences, part 23: Linnaeus and the economy of nature". Bulletin of the Ecological Society of America 88 (1): 72–88. doi:10.1890/0012-9623(2007)88[72:AHOTES]2.0.CO;2. ISSN 0012-9623.
- ↑ 225.0 225.1 Kormandy, E. J.; Wooster, Donald (1978). "Review: Ecology/economy of nature—synonyms?". Ecology 59 (6): 1292–1294. doi:10.2307/1938247. JSTOR 1938247.
- ↑ May, R. (1999). "Unanswered questions in ecology". Philosophical Transactions of the Royal Society B 354 (1392): 1951–1959. doi:10.1098/rstb.1999.0534. PMC 1692702. PMID 10670015.
- ↑ Darwin, Charles (1859). On the Origin of Species (1st ed.). London, UK: John Murray. p. 1. ISBN 0-8014-1319-2.
- ↑ Meysman, F. J. R.; Middelburg, Jack J.; Heip, C. H. R. (2006). "Bioturbation: A fresh look at Darwin's last idea". Trends in Ecology and Evolution 21 (22): 688–695. doi:10.1016/j.tree.2006.08.002. PMID 16901581.
- ↑ Hector, A.; Hooper, R. (2002). "Darwin and the first ecological experiment". Science 295 (5555): 639–640. doi:10.1126/science.1064815. PMID 11809960.
- ↑ Acot, P. (1997). "The Lamarckian cradle of scientific ecology". Acta Biotheoretica 45 (3–4): 185–193. doi:10.1023/A:1000631103244.
- ↑ Forbes, S. (1887). "The lake as a microcosm". Bulletin of the Scientific Association (Peoria, IL): 77–87.
- ↑ 232.0 232.1 Hunt, Caroline Louisa (1912). The life of Ellen H. Richards (1st ed.). Boston: Whitcomb & Barrows.
- ↑ Clements, F. E. (1905). Research methods in ecology. Lincoln, Neb.: University Pub. Comp. ISBN 0-405-10381-6.
- ↑ Simberloff, D. (1980). "A succession of paradigms in ecology: Essentialism to materialism and probalism". Synthese 43: 3–39. doi:10.1007/BF00413854.
- ↑ Gleason, H. A. (1926). "The individualistic concept of the plant association". Bulletin of the Torrey Botanical Club 53 (1): 7–26. doi:10.2307/2479933. JSTOR 2479933.
- ↑ Foster, J. B.; Clark, B. (2008). "The sociology of ecology: ecological organicism versus ecosystem ecology in the social construction of ecological science, 1926–1935". Organization & Environment 21 (3): 311–352. doi:10.1177/1086026608321632.
- ↑ Allee, W. C. (1932). Animal Life and Social Growth. Baltimore: The Williams & Wilkins Company and Associates.
- ↑ Cook, R. E. (1977). "Raymond Lindeman and the trophic-dynamic concept in ecology". Science 198 (4312): 22–26. Bibcode:1977Sci...198...22C. doi:10.1126/science.198.4312.22. PMID 17741875.
- ↑ Odum, E. P. (1968). "Energy flow in ecosystems: A historical review". American Zoologist 8 (1): 11–18. JSTOR 3881528.
- ↑ 240.0 240.1 Ghilarov, A. M. (1995). "Vernadsky's biosphere concept: an historical perspective". The Quarterly Review of Biology 70 (2): 193–203. doi:10.1086/418982. JSTOR 3036242.
- ↑ Itô, Y. (1991). "Development of ecology in Japan, with special reference to the role of Kinji Imanishi". Journal of Ecological Research 6 (2): 139–155. doi:10.1007/BF02347158.
- ↑ Carson, R. (2002). Silent Spring. Houghton Mifflin Company. p. 348. ISBN 0-618-24906-0.
- ↑ 243.0 243.1 243.2 Palamar, C. R. (2008). "The justice of ecological restoration: Environmental history, health, ecology, and justice in the United States". Human Ecology Review 15 (1): 82–94.
- ↑ Krebs, J. R.; Wilson, J. D.; Bradbury, R. B.; Siriwardena, G. M. (1999). "The second Silent Spring". Nature 400 (6745): 611–612. Bibcode:1999Natur.400..611K. doi:10.1038/23127.
- ↑ Eastwood, R. (2004). "Successive replacement of tending ant species at aggregations of scale insects (Hemiptera: Margarodidae and Eriococcidae) on Eucalyptus in south-east Queensland". Australian Journal of Entomology 43: 1–4. doi:10.1111/j.1440-6055.2003.00371.x.
- ↑ Friedman, J.; Harder, L. D. (2004). "Inflorescence architecture and wind pollination in six grass species". Functional Ecology 18 (6): 851–860. doi:10.1111/j.0269-8463.2004.00921.x.
- ↑ Harder, L. D.; Johnson, S. D. (2009). "Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation". New Phytologist 183 (3): 530–545. doi:10.1111/j.1469-8137.2009.02914.x. PMID 19552694.
- ↑ Kiessling, W.; Simpson, C.; Foote, M. (2009). "Reefs as cradles of evolution and sources of biodiversity in the Phanerozoic". Science 327 (5962): 196–198. Bibcode:2010Sci...327..196K. doi:10.1126/science.1182241. PMID 20056888.
- ↑ Sinclair, G. (1826). "On cultivating a collection of grasses in pleasure-grounds or flower-gardens, and on the utility of studying the Gramineae". London Gardener's Magazine 1 (New-Street-Square: A. & R. Spottiswoode). p. 115.
- ↑ Stuart-Fox, D.; Moussalli, A. (2008). "Selection for social signalling drives the evolution of chameleon colour change". PLoS Biology 6 (1): e25. doi:10.1371/journal.pbio.0060025. PMC 2214820. PMID 18232740.
External links
| Find more about Ecology at Wikipedia's sister projects | |
| |
Definitions and translations from Wiktionary |
| |
Media from Commons |
| |
Quotations from Wikiquote |
| |
Textbooks from Wikibooks |
| |
Learning resources from Wikiversity |
- Ecology (Stanford Encyclopedia of Philosophy)
- The Nature Education Knowledge Project: Ecology
- Ecology Journals List of ecological scientific journals
- Ecology Dictionary - Explanation of Ecological Terms
- Basic Terms of Ecology
- Canadian Society for Ecology and Evolution
- Ecological Society of America
- Ecology Global Network
- Ecological Society of Australia
- British Ecological Society
- Ecological Society of China
- International Society for Ecological Economics
- European Ecological Federation
- UN Millennium Ecosystem Assessment
- The Encyclopedia of Earth – Wilderness: Biology & Ecology
- Ecology and Society - A journal of integrative science for resilience and sustainability
- Science Aid: Ecology, U.K. High School (GCSE, Alevel) Ecology
| ||||||||||||||||||||
| ||||||||||||||||||||