Eclox
Eclox, which stands for Enhanced ChemiLuminescence and OXyradical test for water quality analysis, is a rapid toxicity technology that has been shown to correlate with other established toxicity tests. Rapid toxicity testing is unable to identify specific contaminants or their concentrations and instead function as a screening tool to quickly determine whether water is potentially toxic.[1][2][3]
Eclox ECL Assay
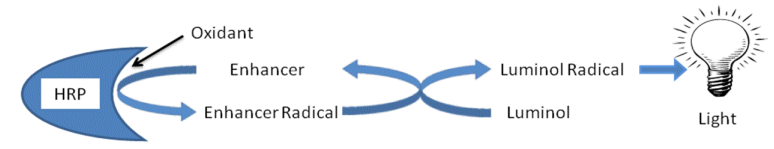
Eclox ECL is a broadband enhanced chemiluminescence (ECL) assay that can be used to qualitatively assess a water sample to determine whether it has been contaminated. ECL reactions are used in a number of clinical and analytical applications and are based upon the oxidation of luminol in the presence of the enzyme horseradish peroxidase (HRP), an oxygen source, and an enhancer such as 4-iodophenol.[4]
Eclox 3[6]
References
- ↑ EPA, 2005. Technologies and Techniques for Early Warning Systems to Monitor and Evaluate Drinking Water Quality: A State-of-the-Art Review
- ↑ Dewhurst, R.E., Wheeler, J.R., Chummun, K.S., Mather, J.D., Callaghan, A., Crane, M., 2002. The Comparison of Rapid Bioassays for the Assessment of Urban Groundwater Quality. Chemosphere 47, 547-554
- ↑ van der Schalie, W.H., James, R.R., Gargan, L., 2006. Selection of a battery of rapid toxicity sensors for drinking water evaluation. Biosensors and Bioelectronics 22, 18-27.
- ↑ Bowman, J.R., 2010. Reliability of the Eclox Enhanced Chemiluminescence Assay for Rapid Field Testing of Drinking Water, Thesis, Texas A&M University System Health Science Center
- ↑ Whitehead, T.P., Thorpe, G.H.G., Maxwell, S.R.J., 1992. Enhanced chemiluminescent assay for antioxidant capacity in biological fluids. Analynca Chumca Acta, 266, 265-277
- ↑ Sawcer, K.E., Thorpe, G.H.G., 2000. The Enhanced Chemiluminescence (ECL) Water Quality Test: Modes of Action and Mechanistic Studies of Substances acting on the Enhanced Chemiluminescence Reaction. R&D Technical Report E86. Bristol, Environmental Agency