Dutasteride
 | |
|---|---|
| Systematic (IUPAC) name | |
| (5α, 17β)-N-{2,5-bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide | |
| Clinical data | |
| Trade names | Avodart |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a603001 |
| Pregnancy cat. | X (US) Not to be handled by pregnant women |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 99% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Half-life | 5 weeks |
| Excretion | Fecal |
| Identifiers | |
| CAS number | 164656-23-9 |
| ATC code | G04CB02 |
| PubChem | CID 6918296 |
| DrugBank | DB01126 |
| ChemSpider | 5293502 |
| UNII | O0J6XJN02I |
| KEGG | D03820 |
| ChEBI | CHEBI:521033 |
| ChEMBL | CHEMBL1200969 |
| Chemical data | |
| Formula | C27H30F6N2O2 |
| Mol. mass | 528.53 g/mol |
| SMILES
| |
| |
| | |
Dutasteride (trademark name Avodart, manufactured by GlaxoSmithKline) is a dual 5-α reductase inhibitor that inhibits conversion of testosterone to dihydrotestosterone (DHT). Used for benign prostatic hyperplasia; however, it increases the risk of erectile dysfunction[1] and decreased sexual desire.[2]
Medical uses

Dutasteride useful for the symptoms of benign prostatic hyperplasia (BPH); colloquially known as an "enlarged prostate".[3]
In those who are being regularly screened 5-alpha-reductase inhibitor (finasteride and dutasteride) reduce the overall risk of being diagnosed with prostate cancer however there is insufficient data to determine if they have an effect on the risk of death and may increase the chance of more serious cases.[4]
Adverse effects
Sexual effects
This class of medications increases rates of erectile dysfunction (with between 5% and 9% developing problems after starting their use).[1] This is linked to lower quality of life and can cause stress in relationships.[1] There is also an association with lowered sexual desire.[2] It has been reported that these adverse sexual side effects may persist even after discontinuation of the drug.[2]
Prostate cancer
The FDA has added a warning to dutasteride about an increased risk of high-grade prostate cancer.[5] While the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer.[6]
Contraindications
Children and women who are or may become pregnant, and people with known significant hypersensitivity (e.g., serious skin reactions, angioedema) to dutasteride or finasteride should not take dutasteride. Exposure to dutasteride and other 5-alpha reductase inhibitors during pregnancy can cause birth defects. Since these medications are readily absorbed through the skin, women who are or may become pregnant should not handle them and if they come into contact with leaking capsules, the contact area should be washed immediately in soapy water. People taking dutasteride should not donate blood and, due to its long half-life, should also not donate blood for at least 6 months after the cessation of treatment.[7]
Mechanism of action
Dutasteride belongs to a class of drugs called 5-alpha-reductase inhibitors, which block the action of the 5-alpha-reductase enzymes that convert testosterone into dihydrotestosterone (DHT).
Dutasteride versus finasteride
Finasteride is also approved for the treatment of benign prostatic hyperplasia, or BPH. The medications belong to the same class of drugs. Dutasteride inhibits two of the three isoforms of 5-alpha reductase, I and II, whereas finasteride only inhibits type II, and has a much shorter half-life.
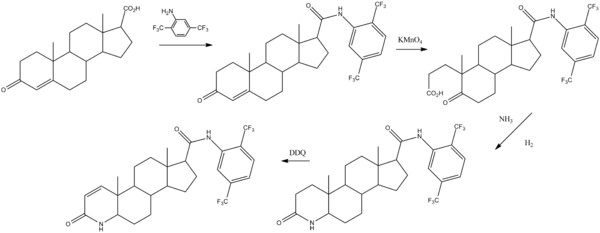
Chemical synthesis
Dutasteride can be synthesized from 3-oxoandrost-4-ene-17-carboxylic acid:[8]
Society and culture
Dutasteride is approved by the Food and Drug Administration (FDA) for treatment of benign prostatic hyperplasia.
References
- ↑ 1.0 1.1 1.2 Gur, S; Kadowitz, PJ; Hellstrom, WJ (January 2013). "Effects of 5-alpha reductase inhibitors on erectile function, sexual desire and ejaculation.". Expert opinion on drug safety 12 (1): 81–90. PMID 23173718.
- ↑ 2.0 2.1 2.2 Traish, AM; Hassani, J; Guay, AT; Zitzmann, M; Hansen, ML (March 2011). "Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients.". The journal of sexual medicine 8 (3): 872–84. PMID 21176115.
- ↑ Slater, S; Dumas, C; Bubley, G (March 2012). "Dutasteride for the treatment of prostate-related conditions.". Expert opinion on drug safety 11 (2): 325–30. PMID 22316171.
- ↑ Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS (2008). "Five-alpha-reductase Inhibitors for prostate cancer prevention". Cochrane Database Syst Rev (2): CD007091. doi:10.1002/14651858.CD007091. PMID 18425978.
- ↑ 5-alpha reductase inhibitors (5-ARIs): Label Change - Increased Risk of Prostate Cancer | U.S. Department of Health & Human Services
- ↑ Walsh, PC (Apr 1, 2010). "Chemoprevention of prostate cancer.". The New England Journal of Medicine 362 (13): 1237–8. doi:10.1056/NEJMe1001045. PMID 20357287.
- ↑ "FDA prescribing information". June 2011. Retrieved 15 September 2013.
- ↑ Batchelor, K. W.; Frye, S. V.; Dorsey, G. F.; Mook, R. A.; 1996, U.S. Patent 5,565,467
External links
| |||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||