Dispersity


In physical and organic chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud,[1] crystals in a rock,[2] or polymer molecules in a solvent.[3] Polymers can possess a distribution of molecular mass; particles often possess a wide distribution of size, surface area and mass; and thin films can possess a varied distribution of film thickness.[citation needed]
IUPAC has deprecated the use of the term polydispersity index having replaced it with the term dispersity, represented by the symbol Đ (pronounced D-stroke[4]) which can refer to either molecular mass or degree of polymerization. It can be calculated using the equation ĐM = Mw/Mn, where Mw is the weight-average molar mass and Mn is the number-average molar mass. It can also be calculated according to degree of polymerization, where ĐX = Xw/Xn, where Xw is the weight-average degree of polymerization and Xn is the number-average degree of polymerization. In certain limiting cases where ĐM = ĐX, it is simply referred to as Đ. IUPAC has also deprecated the terms monodisperse, which is considered to be self-contradictory, and polydisperse, which is considered redundant, preferring the terms uniform and non-uniform instead.[4]
Overview
ĐM = Mw/Mn
where Mw is the weight-average molar mass and
Mn is the number-average molar mass.
A uniform polymer (formerly described as a monodisperse polymer) is composed of molecules of the same mass.[5] Natural polymers are typically uniform.[6] Synthetic uniform polymer chains can be made by processes such as anionic polymerization, a method using an anionic catalyst to produce chains that are similar in length. This technique is also known as living polymerization. It is used commercially for the production of block copolymers. Uniform collections can be easily created through the use of template-based synthesis, a common method of synthesis in nanotechnology.[citation needed]
A polymer material is denoted by the term non-uniform (formerly 'polydisperse') if its chain lengths vary over a wide range of molecular masses. This is characteristic of man-made polymers.. Natural organic matter produced by the decomposition of plants and wood debris in soils (humic substances) also has a pronounced non-uniform character. It is the case of humic acids and fulvic acids, natural polyelectrolyte substances having respectively higher and lower molecular masses. A rather different meaning of polydispersity index is explained in the article Dynamic light scattering (cumulant method subheading). In this sense, the PDI values are in the range from 0 to 1.
The dispersity (Đ, formerly known as polydispersity index, PDI, or heterogeneity index), is a measure of the distribution of molecular mass in a given polymer sample. The dispersity calculated is the weight average molecular weight (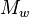 ) divided by the number average molecular weight (
) divided by the number average molecular weight (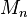 ). It indicates the distribution of individual molecular masses in a batch of polymers. The dispersity has a value equal to or greater than 1, but as the polymer chains approach uniform chain length, Đ approaches unity (1).[7] For some natural polymers, Đ is almost taken as unity.
The dispersity from polymerization is often denoted as Đ
). It indicates the distribution of individual molecular masses in a batch of polymers. The dispersity has a value equal to or greater than 1, but as the polymer chains approach uniform chain length, Đ approaches unity (1).[7] For some natural polymers, Đ is almost taken as unity.
The dispersity from polymerization is often denoted as Đ  ,
where
,
where 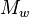 is the weight average molecular weight and
is the weight average molecular weight and 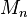 is the number average molecular weight.
is the number average molecular weight. 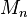 is more sensitive to molecules of low molecular mass, while
is more sensitive to molecules of low molecular mass, while 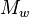 is more sensitive to molecules of high molecular mass.
is more sensitive to molecules of high molecular mass.
Effect of polymerization mechanism
Typical dispersities vary based on the mechanism of polymerization and can be affected by a variety of reaction conditions. In synthetic polymers, it can vary greatly due to reactant ratio, how close the polymerization went to completion, etc. For typical addition polymerization, Đ can range around 10 to 20. For typical step polymerization, most probable values of Đ are around 2 —Carothers' equation limits Đ to values of 2 and below.
Living polymerization, a special case of addition polymerization, leads to values very close to 1. Such is the case also in biological polymers, where the dispersity can be very close or equal to 1, indicating only one length of polymer is present.
Determination methods
- Gel permeation chromatography (also known as size exclusion chromatography)
- Light scattering measurements such as dynamic light scattering
- Direct measurement via mass spectrometry using MALDI or ESI-MS
References
- ↑ Martins, J. A.; Silva Dias, M. A. F. (2009). "The impact of smoke from forest fires on the spectral dispersion of cloud droplet size distributions in the Amazonian region". Environmental Research Letters 4: 015002. doi:10.1088/1748-9326/4/1/015002.
- ↑ Higgins, Michael D. (2000). "Measurement of crystal size distributions". American Mineralogist 85: 1105–1116.
- ↑ Okita, K.; Teramoto, A.; Kawahara, K.; Fujita, H. (1968). "Light scattering and refractometry of a monodisperse polymer in binary mixed solvents". The Journal of Physical Chemistry 72: 278. doi:10.1021/j100847a053.
- ↑ 4.0 4.1 Stepto, R. F. T.; Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl P. (2009). "Dispersity in Polymer Science" Pure Appl. Chem. 81 (2): 351–353. DOI:10.1351/PAC-REC-08-05-02.
- ↑ "monodisperse polymer (See: uniform polymer)". IUPAC Gold Book. International Union of Pure and Applied Chemistry. Retrieved 25 January 2012.
- ↑ Brown, William H.; Foote, Christopher S.; Iverson, Brent L.; Anslyn, Eric V. (2012). Organic chemistry (6 ed.). Cengage Learning. p. 1161. ISBN 978-0-8400-5498-2.
- ↑ Peter Atkins and Julio De Paula, Atkins' Physical Chemistry, 9th edition (Oxford University Press, 2010, ISBN 978-0-19-954337-3)