Diphenyl diselenide
| Diphenyl diselenide | |
|---|---|
 | |
 | |
| IUPAC name Diphenyl diselenide | |
| Other names Phenyl diselenide | |
| Identifiers | |
| CAS number | 1666-13-3 |
| PubChem | 15460 |
| ChemSpider | 14710 |
| RTECS number | JM9152500 |
| Jmol-3D images | {{#if:c1ccc(cc1)[Se][Se]c2ccccc2|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C12H10Se2 |
| Molar mass | 312.13 g mol−1 |
| Appearance | Orange powder |
| Density | 1.84 g/cm3 |
| Melting point | 59-61 °C |
| Solubility in water | Insoluble |
| Solubility in other solvents | Dichloromethane |
| Structure | |
| Coordination geometry |
90° at Se[citation needed] C2 symmetry[citation needed] |
| Dipole moment | 0 D |
| Hazards | |
| R-phrases | R23/25 R33 R50/53 |
| S-phrases | S20/21 S28 S45 S60 S61 |
| Main hazards | Toxic |
| Related compounds | |
| Related compounds | Ph2S2, C6H5SeH |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2 This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
Ph2Se2 is prepared by the oxidation of benzeneselenoate, the conjugate base of benzeneselenol which is generated via the Grignard reagent:[1]
The molecule has idealized C2-symmetry, like hydrogen peroxide and related molecules. The Se-Se bond length of 2.29 Å the C-Se-Se-C dihedral angle is 82° and the C-Se-Se angles are near 110°.[2]
Reactions
One reactions characteristic of Ph2Se2 is its reduction:
- Ph2Se2 + 2 Na → 2 PhSeNa
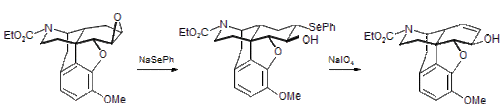
PhSeNa is a useful nucleophile used to introduce the phenylselenyl group by nucleophilic substitution of alkyl halides, alkyl sulfonates (mesylates or tosylates) and epoxides. The example below was taken from a synthesis of morphine.[3]
Another characteristic reaction is chlorination:
- Ph2Se2 + Cl2 → 2 PhSeCl
PhSeCl is a powerful electrophile, used to introduce PhSe groups by reaction with a variety of nucleophiles, including enolates, enol silyl ethers, Grignard reagents, organolithium reagents, alkenes and amines. In the sequence below (early steps in the synthesis of Strychnofoline), a PhSe group is introduced by reaction of a lactam enolate with PhSeCl.[4] This sequence is a powerful method for the conversion of carbonyl compounds to their α,β-unsaturated analogs.[5]
Diphenyl diselenide itself is also a source of a weakly electrophilic PhSe group in reactions with relatively powerful nucleophiles like Grignard reagents, lithium reagents and ester enolates (but not ketone enolates or weaker nucleophiles). PhSeCl is both more reactive, and more efficient, since with Ph2Se2 half of the selenium is wasted.
- Ph2Se2 + Nu− → PhSeNu + PhSe−
N-Phenylselenophtalimide (N-PSP) can be used if PhSeCl is too strong and diphenyl diselenide is too weak or wasteful.[6]
Safety
Organoselenium compounds are toxic.
References
- ↑ Reich, H. J.; Cohen, M. L.; Clark, P. S. (1979), "Reagents for Synthesis of Organoselenium Compounds: Diphenyl Diselenide and Benzeneselenenyl Chloride", Org. Synth. 59: 141; Coll. Vol. 6: 533
- ↑ Marsh, R. E. (1952). "The Crystal Structure of Diphenyl Diselenide". Acta Crystallographica 5 (4): 458–462. doi:10.1107/S0365110X52001349.
- ↑ Taber, D. F.; Neubert, T. D.; Rheingold, A. L. (2002). "Synthesis of (−)-Morphine". Journal of the American Chemical Society 124 (42): 12416–12417. doi:10.1021/ja027882h. PMID 12381175.
- ↑ Lerchner, A.; Carreira, E. M. (2002). "First Total Synthesis of (±)-Strychnofoline via a Highly Selective Ring-Expansion Reaction". Journal of the American Chemical Society 124 (50): 14826–14827. doi:10.1021/ja027906k.
- ↑ Reich, H. J.; Wollowitz, S. (1993). "Preparation of α,β-Unsaturated Carbonyl Compounds and Nitriles by Selenoxide Elimination". Organic Reactions 44: 1–296. doi:10.1002/0471264180.or044.01.
- ↑ Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R.; Corttés, M.; Armstrong, V. (1999). "Synthesis and Antitumor Activity of Puupehedione and Related Compounds". Tetrahedron 55 (52): 15181–15208. doi:10.1016/S0040-4020(99)00992-8.