Dimethyl disulfide
| Dimethyl disulfide | |
|---|---|
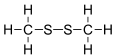 |
|
 |
 |
| IUPAC name (Methyldisulfanyl)methane | |
| Other names Dimethyl disulphide; Methyl disulfide; Methyldisulfide; Dimethyldisulfide; Methyldithiomethane; 2,3-Dithiabutane | |
| Identifiers | |
| Abbreviations | DMDS |
| CAS number | 624-92-0 |
| PubChem | 12232 |
| ChemSpider | 11731 |
| ChEBI | CHEBI:4608 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C2H6S2 |
| Molar mass | 94.20 g mol−1 |
| Appearance | Colorless to yellowish liquid[1] |
| Density | 1.06 g/cm3[1] |
| Melting point | −85 °C; −121 °F; 188 K ([1]) |
| Boiling point | 110 °C; 230 °F; 383 K ([1]) |
| Solubility in water | 2.5 g/L (20 °C)[1] |
| Vapor pressure | 3.8 kPa (at 25 °C) Arkema data sheet |
| Hazards | |
| Flash point | 15 °C (59 °F)[1] |
| Autoignition temperature | 370 °C (698 °F)[1] |
| LD50 | 190 mg/kg (oral, rat)[2] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Dimethyl disulfide (DMDS) is an organic chemical compound with the molecular formula CH3SSCH3 which is the simplest disulfide. It is a flammable liquid with an unpleasant, garlic-like odor.
Occurrence
Dimethyl disulfide along with dimethyl sulfide and dimethyl trisulfide have been confirmed as volatile compounds given off by the fly-attracting plant known as dead-horse arum (Helicodiceros muscivorus). These flies are attracted to the odor of fetid meat, they help pollinate this plant.[3]
DMDS can be produced by the oxidation of methanethiol, e.g. with iodine:
- 2 CH3SH + I2 → CH3SSCH3 + 2 HI
Chemical reactions
Important reactions include chlorination giving methanesulfenyl chloride (CH3SCl), methanesulfinyl chloride (CH3S(O)Cl),[4] and methanesulfonyl chloride (CH3SO2Cl) as well as oxidation with hydrogen peroxide or peracetic acid giving the thiosulfinate methyl methanethiosulfinate (CH3S(O)SCH3).[5]
Uses
DMDS is used as a food additive in onion, garlic, cheese, meats, soups, savory flavors, and fruit flavors.[6] Industrially, DMDS is used in oil refineries as a sulfiding agent.[7] DMDS is also an effective soil fumigant in agriculture, registered in many states in the U.S. as well as globally. In this capacity, DMDS is an important alternative in replacing methyl bromide, which is being phased out. This pesticide is marketed as "Paladin" by Arkema.[8][9]
Food use
DMDS is used to alpha substitute 2-methylfuran-acrolein to produce a food stuff used in concentrations from 0.02 ppm up to 50 ppm.
Industrial use
DMDS is a stable pale yellow liquid which works as an effective product in the sulfiding process because of its high sulfur content. It is the sulfiding industry's reagent of choice because it offers more sulfur per pound of reagent when compared to its nearest competitor dimethyl sulfide.[10]
DMDS also works as an effective product for operators in the petrochemicals industry who must protect their steam-cracking coils against the formation of coke and carbon monoxide.
DMDS is used in the preparation of 4-(methylthio)phenol which is used in the production of various pesticides. DMDS and chlorine are reacted with borontriflouride phenolate to produce 4-(methylthio)phenol. Thiophene and DMDS are blended with combustible hydrocarbon fuel gas to impart a gassy odor to the fuel gas. DMDS is used as a sulfiding reagent to control catalyst activity.[11]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Record in the GESTIS Substance Database from the IFA
- ↑ , EPA DMDS Fact Sheet
- ↑ Marcus C. Stensmyr, Isabella Urru, Ignazio Collu, Malin Celander, Bill S. Hansson, Anna-Maria Angioy (2002). "Rotting smell of dead-horse arum florets". Nature 420 (6916): 625–626. doi:10.1038/420625a. PMID 12478279.
- ↑ Irwin B. Douglass and Richard V. Norton "Methanesulfinyl Chloride" Organic Syntheses, Coll. Vol. 5, p.709-712 (1973).
- ↑ Block, Eric; O'Connor, John (1974). "Chemistry of alkyl thiosulfinate esters. VI. Preparation and spectral studies". Journal of the American Chemical Society 96 (12): 3921. doi:10.1021/ja00819a033.
- ↑ , OSHA
- ↑ Dimethyl Disulfide (DMDS), Arkema, Inc.
- ↑ "DMDS for agricultural soil fumigation". Arkema. Retrieved 2013-09-06.
- ↑ Registration of Paladin and Paladin EC containing the new active ingredient dimethyl disulfide. New York State Department of Environmental Conservation. March 9, 2012.
- ↑ Dimethyl Disulfide (DMDS)
- ↑ CAS Database Reference, Detailed Methyl Disulfide Applications: Food Use and Industrial Use