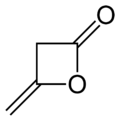
Diketene
| Diketene | |
|---|---|
 |
 |
| IUPAC name 4-methylideneoxetan-2-one | |
| Other names γ-methylenebutyrolactone | |
| Identifiers | |
| CAS number | 674-82-8 |
| ChemSpider | 12140 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C4H4O2 |
| Molar mass | 84.08 g mol−1 |
| Density | 1.09 g cm−3 |
| Melting point | −7 °C |
| Boiling point | 127 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Diketene is an organic compound formed by dimerization of ethenone (ketene). Diketene is a member of the oxetane family. It is used as a chemical reagent in organic chemistry.[1] It is a colorless liquid and heating regenerates the ketene monomer. Alkylated ketenes also dimerize with ease and form substituted diketenes.
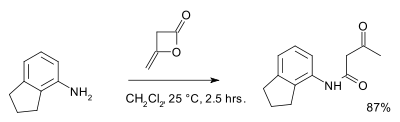
Diketene readily hydrolyses in water forming acetoacetic acid, its half-life in pure water is approximately 45 minutes a 25 °C at 2<pH<7.[2] Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivatives. An example is the reaction with 2-aminoindane:[3]
Despite its high reactivity as an alkylating agent, and unlike analogue β-lactones propiolactone and β-butyrolactone, diketene is inactive as a carcinogen, possibly due to the instability of its DNA adducts.[4]
Diketene is an important industrial intermediate used for the production of acetoacetate esters and amides as well as substituted 1-phenyl-3-methylpyrazolones. The latter are used in the manufacture of dyestuffs and pigments.[5]
Certain diketenes with two aliphatic chains, such as alkyl ketene dimer (AKD), are used industrially to improve hydrophobicity in paper.
References
- This article incorporates information from the German Wikipedia.
- ↑ Beilstein E III/IV 17: 4297.
- ↑ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Kinetic Study of the Neutral and Base Hydrolysis of Diketene". Phys. Org. Chem 22 (5): n/a. doi:10.1002/poc.1483.
- ↑ Kiran Kumar Solingapuram Sai, Thomas M. Gilbert, and Douglas A. Klumpp (2007). "Knorr Cyclizations and Distonic Superelectrophiles". J. Org. Chem. 72 (25): 9761–9764. doi:10.1021/jo7013092. PMID 17999519.
- ↑ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Chemical Reactivity and Biological Activity of Diketene". Chem. Res. Toxicol. 21 (10): 1964–1969. doi:10.1021/tx800153j. PMID 18759502.
- ↑ Ashford's Dictionary of Industrial Chemicals, Third Edition, 2011, pages 3241-2.