Diethylmercury
From Wikipedia, the free encyclopedia
| Diethylmercury | ||
|---|---|---|
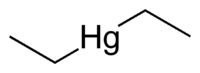 | ||
| IUPAC name diethylmercury | ||
| Identifiers | ||
| CAS number | 627-44-1 | |
| Properties | ||
| Molecular formula | C4H10Hg | |
| Molar mass | 258.71 g/mol | |
| Appearance | Colorless liquid | |
| Density | 2.446 g/ml | |
| Melting point | -45 °C | |
| Boiling point | 156 - 157 °C | |
| Solubility in water | Insoluble | |
| Viscosity | ? cP at ? °C | |
| Hazards | ||
| R-phrases | R26, R27, R28, R33, R50, R53 | |
| S-phrases | S13, S28, S36, S45, S60, S61 | |
| Flash point | N/A | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.[1] This chemical can cross the blood–brain barrier, causing permanent brain damage.
See also
- Dimethylmercury, a related compound
- Ethylmercury
- Mercury poisoning
References
| |||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.