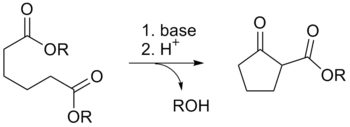Dieckmann condensation
From Wikipedia, the free encyclopedia
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters.[1][2][3][4][5] It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation.
Reaction Mechanism
The acidic hydrogen between the two carbonyl groups is deprotonated in step four . Protonation with a Brønsted-Lowry acid (H3O+ for example) re-forms the β-keto ester. [6] This deprotonation step is the driving force for this reaction.
Owing to the steric stability of five- and six-membered ring structures, these will preferentially be formed. 1,4- and 1,6 diesters will form five-membered cyclic β-keto esters, while 1,5- and 1,7 diesters will form six-membered β-keto esters.[7]
See also
- Claisen condensation
- Gabriel-Colman rearrangement
References
- ↑ Dieckmann, W. Ber. 1894, 27, 102 & 965
- ↑ Dieckmann, W. Ber. 1900, 33, 595 & 2670
- ↑ Dieckmann, W. Ann. 1901, 317, 51 & 93
- ↑ Schaefer, J. P.; Bloomfield, J. J. (1967). "The Dieckmann Condensation (Including the Thorpe-Ziegler Condensation)". Organic Reactions 15: 1–203. doi:10.1002/0471264180.or015.01.
- ↑ Davis, B. R.; Garrett, P. J. Comp. Org. Syn. 1991, 2, 806-829. (Review)
- ↑ Janice Gorzynski Smith (2007). Organic Chemistry (2nd ed.). pp. 932–933. ISBN 978-0073327495.
- ↑ "Dieckmann Condensation". Organic Chemistry Portal.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.