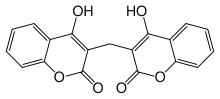
Dicoumarol
 | |
|---|---|
| Systematic (IUPAC) name | |
| 3,3'-methylenebis(4-hydroxy-2H-chromen-2-one) | |
| Clinical data | |
| MedlinePlus | a605015 |
| Legal status | ℞-only (US) |
| Pharmacokinetic data | |
| Protein binding | plasmatic proteins |
| Metabolism | hepatic |
| Excretion | faeces, urine |
| Identifiers | |
| CAS number | 66-76-2 |
| ATC code | B01AA01 |
| PubChem | CID 653 |
| DrugBank | DB00266 |
| ChemSpider | 10183330 |
| UNII | 7QID3E7BG7 |
| KEGG | D03798 |
| ChEBI | CHEBI:4513 |
| ChEMBL | CHEMBL1466 |
| NIAID ChemDB | 016070 |
| Chemical data | |
| Formula | C19H12O6 |
| Mol. mass | 336.295 g/mol |
| SMILES
| |
| |
| | |
Dicoumarol (INN) or dicumarol (USAN) is a naturally occurring anticoagulant that functions as a functional vitamin K depleter (similar to warfarin, a drug that dicoumarol inspired). It is also used in biochemical experiments as an inhibitor of reductases.
Dicoumarol is a natural chemical substance of combined plant and fungal origin. It is a derivative of coumarin, a bitter tasting but sweet-smelling substance made by plants that does not itself affect coagulation, but which is (classically) transformed in mouldy feeds or silages by a number of species of fungi, into active dicoumarol. Dicoumarol does affect coagulation, and was discovered in mouldy wet sweet-clover hay, as the cause of a naturally occurring bleeding disease in cattle.[1] See warfarin for a more detailed discovery history.
Identified in 1940, dicoumarol became the prototype of the 4-hydroxycoumarin derivative anticoagulant drug class. Dicoumarol itself, for a short time, was employed as a medicinal anticoagulant drug, but since the mid-1950s has been replaced by its simpler derivative warfarin, and other 4-hydroxycoumarin drugs.
It is given orally, and it acts within two days.
Mechanism of action
Like all 4-hydroxycoumarin drugs it is a competitive inhibitor of vitamin K epoxide reductase, an enzyme that recycles vitamin K, thus causing depletion of active vitamin K in blood. This prevents the formation of the active form of prothrombin and several other coagulant enzymes.
These compounds are not direct antagonists (in the pharmaceutical sense) of vitamin K itself, but rather act to deplete reduced vitamin K in tissues. For this reason vitamin K antagonizes their effect (rather than the reverse), and this has led to the loose terminology of vitamin K antagonism.
Administration of vitamin K is therefore the antidote for dicoumarol toxicity. The action and toxicity of the drug and the antidote effectiveness are measured with the prothrombin time (PT) blood test.
Uses
Dicoumarol was used along with heparin, for the treatment of deep venous thrombosis. Unlike heparin, this class of drugs may be used for months or years.
References
- ↑ Nicole Kresge, Robert D. Simoni and Robert L. Hill (2005-02-25). "Sweet clover disease and warfarin review". Jbc.org. Retrieved 2012-09-26.
- Cullen J, Hinkhouse M, Grady M, Gaut A, Liu J, Zhang Y, Weydert C, Domann F, Oberley L (2003). "Dicumarol inhibition of NADPH: quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism.". Cancer Res 63 (17): 5513–20. PMID 14500388.
- Mironov A, Colanzi A, Polishchuk R, Beznoussenko G, Mironov A, Fusella A, Di Tullio G, Silletta M, Corda D, De Matteis M, Luini A (2004). "Dicumarol, an inhibitor of ADP-ribosylation of CtBP3/BARS, fragments golgi non-compact tubular zones and inhibits intra-golgi transport.". Eur J Cell Biol 83 (6): 263–79. doi:10.1078/0171-9335-00377. PMID 15511084.
- Abdelmohsen K, Stuhlmann D, Daubrawa F, Klotz L (2005). "Dicumarol is a potent reversible inhibitor of gap junctional intercellular communication.". Arch Biochem Biophys 434 (2): 241–7. doi:10.1016/j.abb.2004.11.002. PMID 15639223.
- Thanos C, Liu Z, Reineke J, Edwards E, Mathiowitz E (2003). "Improving relative bioavailability of dicumarol by reducing particle size and adding the adhesive poly(fumaric-co-sebacic) anhydride.". Pharm Res 20 (7): 1093–100. doi:10.1023/A:1024474609667. PMID 12880296.]
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||