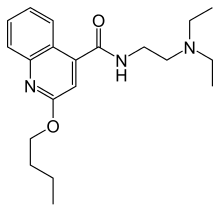
Cinchocaine (INN/BAN) or dibucaine (USAN) is an amide local anesthetic. Among the most potent and toxic of the long-acting local anesthetics, current use of cinchocaine is generally restricted to spinal and topical anesthesia.[1][2] It is sold under the brand names Cincain, Nupercainal, Nupercaine and Sovcaine.
Medical use
Cinchocaine is the active ingredient in some topical hemorrhoid creams such as Proctosedyl. It is also a component of the veterinary drug Somulose, used for euthanasia of horses and cattle.
Physical properties
Cinchocaine is relatively insoluble in alkaline aquatic solutions.
See also
References
- ↑ Martindale, The Extra Pharmacopoeia, 30th ed, p1006
- ↑ Dibucaine
Further reading
- Abdel-Ghani N, Youssef A, Awady M (2005). "Cinchocaine hydrochloride determination by atomic absorption spectrometry and spectrophotometry.". Farmaco 60 (5): 419–24. doi:10.1016/j.farmac.2005.03.001. PMID 15910814.
- Souto-Padron T, Lima AP, de Oliveira Ribeiro R. (2006). "Effects of dibucaine on the endocytic/exocytic pathways in Trypanosoma cruzi.". Parasitol Res 99 (4): 317. doi:10.1007/s00436-006-0192-1. PMID 16612626.
- Nounou M, El-Khordagui L, Khalafallah N (2005). "Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles.". Acta Pol Pharm 62 (5): 369–79. PMID 16459486.
- Aroti,A.;Leontidis, E. (2001). "Simultaneous Determination of the Ionization Constant and the Solubility of Sparingly Soluble Drug Substances. A Physical Chemistry Experiment .". Journal of Chemical Education 78 (6): 786–788. doi:10.1021/ed078p786.
|
|---|
| | Antihemorrhoidals for topical use |
|
|---|
| | Antivaricose therapy |
|
|---|
| | Capillary stabilising agents |
|
|---|
|
|
anat (a:h/u/t/a/l,v:h/u/t/a/l)/phys/devp/cell/prot
|
noco/syva/cong/lyvd/tumr, sysi/epon, injr
|
proc, drug (C2s+n/3/4/5/7/8/9)
|
|
|
|
|
|---|
| | Esters |
|
|---|
| | Amides | |
|---|
| | Combinations | |
|---|
|
|
anat (h/r/t/c/b/l/s/a)/phys (r)/devp/prot/nttr/nttm/ntrp
|
noco/auto/cong/tumr, sysi/epon, injr
| |
|
|
|