Dianin's compound
| Dianin's compound | |
|---|---|
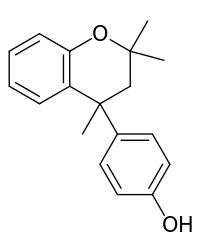 | |
| IUPAC name 4-(2,2,4-Trimethyl-4-chromanyl)phenol | |
| Other names 4-p-Hydroxyphenyl-2,2,4-trimethylchroman | |
| Identifiers | |
| CAS number | 472-41-3 |
| PubChem | 97788 |
| ChemSpider | 88264 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C18H20O2 |
| Molar mass | 268.35 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Dianin's compound (4-p-hydroxyphenyl-2,2,4-trimethylchroman) was invented by Aleksandr Dianin in 1914.[1] This compound is a condensation isomer of bisphenol A and acetone and of special importance in host-guest chemistry because it can form a large variety of clathrates with suitable guest molecules. One example is the clathrate of Dianin's compound with morpholine.[2] Slow evaporation of a solution containing both organic compounds yields crystals. Each asymmetric unit cell making up the crystal contains six chroman molecules of which two are deprotonated and two protonated morpholine molecules. The six chroman molecules are racemate pairs.
References
- ↑ A.P. Dianin, J. Russ. Phys. Chem. Soc., 1914, 36, 1310
- ↑ Gareth O. Lloyd, Martin W. Bredenkamp and Leonard J. Barbour (2005). "Enclathration of morpholinium cations by Dianin's compound: salt formation by partial host-to-guest proton transfer". Chemical Communications 32 (32): 4053–4055. doi:10.1039/b507726e. PMID 16091797.