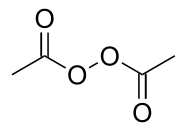
Diacetyl peroxide
| Diacetyl peroxide | |
|---|---|
 | |
 | |
| IUPAC name acetyl ethaneperoxoate | |
| Other names acetylperoxide; diacetyl peroxide; Peroxide, diacetyl; ethanoyl peroxide; acetyl ethaneperoxoate; ethanoyl ethaneperoxoate; peracetic acid acetyl ester | |
| Identifiers | |
| CAS number | 110-22-5 |
| PubChem | 8040 |
| ChemSpider | 7749 |
| EC number | 203-748-8 |
| UN number | 2084 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C4H6O4 |
| Molar mass | 118.09 g mol−1 |
| Appearance | Colorless crystals [1] |
| Density | 1.163 g/cm3[1] |
| Melting point | 30 °C; 86 °F; 303 K |
| Boiling point | 121.4° at 760mmHg, 63° at 21mmHg[2] |
| Solubility in water | slight in cold water [1] |
| Hazards | |
| Main hazards | Explosive |
| NFPA 704 |
 2
1
4
|
| Flash point | 32.2 °C (45 °C [3]) |
| Explosive data | |
| Shock sensitivity | Very high / moderate when wet |
| Friction sensitivity | Very high / moderate when wet |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Diacetyl peroxide is an organic peroxide that is a crystalline, sand-like solid with a sharp odor.[3] The commercial product consists of a 25% solution of acetyl peroxide in dimethyl phthalate.[1]
Reactivity
Peroxides, such as diacetyl peroxide, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides, while strongly reduced material such as sulfides, nitrides, and hydrides may react explosively.
There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness. [4] Pure (100%) material is a severe explosion hazard. It should not be stored after preparation, nor heated above 30 C. [5]
There have been reports of detonation of the pure material. The 25% solution also has explosive potential when inadvertent partial evaporation of a weak solution can create an explosive solution or a shock-sensitive crystalline material. [6] It is essential to prevent the separation of the crystalline peroxide even in traces, since when dry, it is shock sensitive and is a high explosion risk.[7] Application of fluorine to aqueous sodium acetate solution caused an explosion involving the formation of diacetyl peroxide.[7] Dry acetyl peroxide is unpredictable. Five grains of it were removed from an ice chest and detonated violently. Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently.[8] Diacetyl peroxide can be formed when a mixture of hydrogen peroxide, and excess acetic anhydride reacts with one of the reaction products peracetic acid. [9]
Health hazards
Contact with liquid causes irritation of eyes and skin. If ingested, irritates mouth and stomach.[10] [11] [12] [13]
Fire hazards
This compound may explode in a fire, and burn with accelerating intensity.[14]
The threshold quantity for Process Safety Management per Occupational Safety and Health Administration 1910.119 is 5,000 lbs if the concentration of the diacetyl peroxide solution is greater than 70%. [15]
References
- ↑ 1.0 1.1 1.2 1.3 Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 13.
- ↑ Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 79th ed. Boca Raton, FL: CRC Press Inc., 1998-1999., p. 3-250
- ↑ 3.0 3.1 Acetyl peroxide
- ↑ Sax, N.I. Dangerous Properties of Industrial Materials. 4th ed. New York: Van Nostrand Reinhold, 1975., p. 357
- ↑ Hawley, G.G. The Condensed Chemical Dictionary. 9th ed. New York: Van Nostrand Reinhold Co., 1977., p. 10
- ↑ National Research Council. Prudent Practices for Handling Hazardous Chemicals in Laboratories. Washington, DC: National Academy Press, 1981., p. 106
- ↑ 7.0 7.1 Bretherick, L. Handbook of Reactive Chemical Hazards. 4th ed. Boston, MA: Butterworth-Heinemann Ltd., 1990, p. 453, 1104.
- ↑ National Fire Protection Association Fire Protection Guide on Hazardous Materials. 7th ed. Boston, Mass.: National Fire Protection Association, 1978., p. 491M-143
- ↑ "Chemical Safety: Synthesis Procedure". Chemical & Engineering News 89 (2): 2. 2011-01-10.
- ↑ National Research Council. Prudent Practices for Handling Hazardous Chemicals in Laboratories. Washington, DC: National Academy Press, 1981., p. 106
- ↑ International Labour Office. Encyclopaedia of Occupational Health and Safety. 4th edition, Volumes 1-4 1998. Geneva, Switzerland: International Labour Office, 1998., p. 104.349
- ↑ Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 545
- ↑ Mackison, F. W., R. S. Stricoff, and L. J. Partridge, Jr. (eds.). NIOSH/OSHA - Occupational Health Guidelines for Chemical Hazards. DHHS(NIOSH) Publication No. 81-123 (3 VOLS). Washington, DC: U.S. Government Printing Office, Jan. 1981., p. 1
- ↑ National Fire Protection Association. Fire Protection Guide on Hazardous Materials. 7th ed. Boston, Mass.: National Fire Protection Association, 1978., p. 49-110
- ↑
- Peroxide, diacetyl (C4H6O4) Landolt-Boernstein Substance/ Property Index
- Zuordnung der Organischen Peroxide zu Gefahrgruppen nach § 3 Abs. 1 (in German), Berufsgenossenschaft Handel und Warendistribution