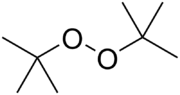
Di-tert-butyl peroxide
| Di-tert-butyl peroxide | |
|---|---|
 | |
 | |
| Identifiers | |
| CAS number | 110-05-4 |
| PubChem | 8033 |
| ChemSpider | 7742 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C8H18O2 |
| Molar mass | 146.23 g mol−1 |
| Density | 0.704 g/cm³ |
| Melting point | −40 °C; −40 °F; 233 K |
| Boiling point | 109 to 111 °C; 228 to 232 °F; 382 to 384 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Di-tert-butyl peroxide or DTBP is an organic compound consisting of a peroxide group flanked by two tert-butyl groups. It is amongst the most stable organic peroxides. The peroxide bond undergoes homolysis at temperatures >100 °C, and for this reason di-tert-butyl peroxide is commonly used as a radical initiator in organic synthesis and polymer chemistry.
This compound will decompose aerobically and also anaerobically, making it a very interesting fuel source.
Decomposition reaction:
Two Canadian scientists, H. O. Pritchard and P. Q. E. Clothier, have demonstrated and suggested the use of DTBP in engines where oxygen is limited, since it will work whether or not oxygen is present.[1]
References
- ↑ H. O. Pritchard and P. Q. E. Clothier (1986), "Anaerobic operation of an internal combustion engine", J. Chem. Soc. Chem. Commun. 1986 (20): 1529–1530, doi:10.1039/C39860001529
External links
- US 5288919, Faraj, Mahmoud K., "Preparation of dialkyl peroxides", published 13 May 1993, issued 22 February 1994
- US 5312998, Liotta, Frank J. (Jr.); Mahmoud K. Faraj & Daniel B. Pourreau et al., "Integrated process for the production of ditertiary butyl peroxide", published 10 June 1993, issued 17 May 1994
- US 5371298, Pourreau, Daniel B.; Haven S. (Jr.) Kesling & Frank J. (Jr.) Liotta et al., "Preparation of dialkyl peroxides", published 22 December 1993, issued 6 December 1994