Dexlansoprazole
 | |
|---|---|
| Systematic (IUPAC) name | |
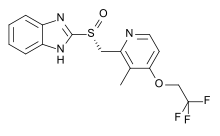
| (R)-(+)2-([3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a695020 |
| Licence data | US FDA:link |
| Pregnancy cat. | B (US) |
| Legal status | ℞-only (US) |
| Routes | Oral |
| Pharmacokinetic data | |
| Excretion | 50% renal and 47% in the feces[1] |
| Identifiers | |
| CAS number | 138530-94-6 |
| ATC code | A02BC06 |
| PubChem | CID 9578005 |
| ChemSpider | 7852369 |
| UNII | UYE4T5I70X |
| KEGG | D08903 |
| ChEMBL | CHEMBL1201863 |
| Chemical data | |
| Formula | C16H14F3N3O2S |
| Mol. mass | 369.363 g/mol |
| SMILES
| |
| |
| | |
Dexlansoprazole (INN, trade names Kapidex, Dexilant) is a proton pump inhibitor that is marketed by Takeda Pharmaceuticals. Chemically, it is an enantiomer of lansoprazole. The compound was launched in the US for use in the treatment and maintenance of patients with erosive oesophagitis and non-erosive gastro-oesophageal reflux disease (GERD or GORD).[2] Dexlansoprazole was approved by the U.S. Food and Drug Administration (FDA) on January 30, 2009.[2]
Pharmacokinetics
After racemic lansoprazole is applied orally, 80% of the circulating drug is dexlansoprazole. Moreover, both enantiomers have a similar effect on the proton pump.[3] Consequently, the main advantage of Kapidex is not the enantiopure substance, but the pharmaceutical formulation.
Kapidex is based on a dual release technology, with the first quick release producing a plasma peak concentration about one hour after application, and the second retarded release producing another peak about four hours later.[2][4] As of November 2009, clinical relevance of this form of release has yet to be shown.
Mechanism of action
Dexlansoprazole selectively inhibits the parietal cell membrane enzyme (H+,K+)-ATPase, typically referred to as the proton pump, thus blocking the final step of acid production.[5]
Adverse effects
Antacid preparations such as dexlansoprazole, by suppressing acid mediated break down of proteins, lead to an elevated risk of developing food and drug allergies. This happens due to undigested proteins then passing into the gastrointestinal tract where sensitisation occurs. It is unclear whether this risk occurs with only long-term use or with short-term use as well.[6]
Naming confusion
Since Kapidex was approved in January 2009, there have been reports of dispensing errors because of confusion with the drugs Casodex (bicalutamide) and Kadian (morphine sulfate), which have very different uses from Kapidex and from each other. On March 4, 2010, the FDA has approved a name change for Kapidex to avoid confusion with the two other medications. Effective in late April 2010, Takeda Pharmaceuticals North America began marketing Kapidex under the new name Dexilant.[7]
References
- ↑ Product Information: DEXILANT(R) delayed release oral capsules, dexlansoprazole delayed release oral capsules. Takeda Pharmaceuticals America, Inc., Deerfield, IL, 2010.
- ↑ 2.0 2.1 2.2 FDA Approves KAPIDEX (dexlansoprazole) delayed release capsules for the Treatment of GERD
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2009.
- ↑ Metz, DC; Vakily, M; Dixit, T; Mulford, D (1 May 2009). "Review article: dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapy". Aliment Pharmacol Ther 29 (9): 928–37. doi:10.1111/j.1365-2036.2009.03984.x. PMID 19298580.
- ↑ Product Information: DEXILANT(R) delayed release oral capsules, dexlansoprazole delayed release oral capsules. Takeda Pharmaceuticals America, Inc., Deerfield, IL, 2010.
- ↑ Pali-Schöll I, Jensen-Jarolim E (April 2011). "Anti-acid medication as a risk factor for food allergy". Allergy 66 (4): 469–77. doi:10.1111/j.1398-9995.2010.02511.x. PMID 21121928.
- ↑ "KAPIDEX (dexlansoprazole) Renamed DEXILANT in U.S. to Avoid Name Confusion". Takeda. 4 March 2010.
External links
- DEXILANT (product's website)
- Takeda Pharmaceuticals (manufacturer's website)
| ||||||||||||||||||||||