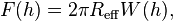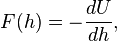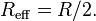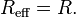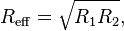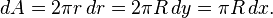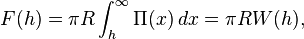Derjaguin approximation
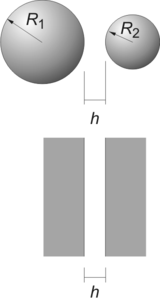
The Derjaguin approximation due to the Russian scientist Boris Derjaguin expresses the force profile acting between finite size bodies in terms of the force profile between two planar semi-infinite walls.[1] This approximation is widely used to estimate forces between colloidal particles, as forces between two planar bodies are often much easier to calculate. The Derjaguin approximation expresses the force F(h) between two bodies as a function of the surface separation as[2]
where W(h) is the interaction energy per unit area between the two planar walls and Reff the effective radius. When the two bodies are two spheres of radii R1 and R2, respectively, the effective radius is given by
Experimental force profiles between macroscopic bodies as measured with the surface forces apparatus (SFA)[3] or colloidal probe technique[4] are often reported as the ratio F(h)/Reff.
Quantities involved and validity
The force F(h) between two bodies is related to the interaction free energy U(h) as
where h is the surface-to-surface separation. Conversely, when the force profile is known, one can evaluate the interaction energy as
When one considers two planar walls, the corresponding quantities are expressed per unit area. The disjoining pressure is the force per unit area and can be expressed by the derivative
where W(h) is the surface free energy per unit area. Conversely, one has
The main restriction of the Derjaguin approximation is that it is only valid at distances much smaller than the size of the objects involved, namely h « R1 and h « R2. Furthermore, it is a continuum approximation and thus valid at distances larger than the molecular length scale. Even when rough surfaces are involved, this approximation has been shown to be valid in many situations.[5] Its range of validity is restricted to distances larger than the characteristic size of the surface roughness features (e.g., root mean square roughness).
Special cases

Frequent geometries considered involve the interaction between two identical spheres of radius R where the effective radius becomes
In the case of interaction between a sphere of radius R and a planar surface, one has
The above two relations can be obtained as special cases of the expression for Reff given further above. For the situation of perpendicularly crossing cylinders as used in the surface forces apparatus, one has
where R1 and R2 are the curvature radii of the two cylinders involved.
Simplified derivation

Consider the force F(h) between two identical spheres of radius R as an illustration. The surfaces of the two respective spheres are thought to be sliced into infinitesimal disks of width dr and radius r as shown in the figure. The force is given by the sum of the corresponding swelling pressures between the two disks
where x is the distance between the disks and dA the area of one of these disks. This distance can be expressed as x=h+2y. By considering the Pythagorean theorem on the grey triangle shown in the figure one has
Expanding this expression and realizing that y « R one finds that the area of the disk can be expressed as
The force can now be written as
where W(h) is the surface free energy per unit area introduced above. When introducing the equation above, the upper integration limit was replaced by infinity, which is approximately correct as long as h « R.
General case
In the general case of two convex bodies, the effective radius can be expressed as follows[6]
where R'i and R"i are the principal radii of curvature for the surfaces i = 1 and 2, evaluated at points of closest approach distance, and φ is the angle between the planes spanned by the circles with smaller curvature radii. When the bodies are non-spherical around the position of closest approach, a torque between the two bodies develops and is given by[6]
where
The above expressions for two spheres are recovered by setting R'i = R"i = Ri. The torque vanishes in this case.
The expression for two perpendicularly crossing cylinders is obtained from R'i = Ri and R"i → ∞. In this case, torque will tend to orient the cylinders perpendicularly for repulsive forces. For attractive forces, the torque will tend to align them.
These general formulas have been used to evaluate approximate interaction forces between ellipsoids.[7]
Beyond the Derjaguin approximation
The Derjaguin approximation is unique given its simplicity and generality. To improve this approximation, the surface element integration method was proposed to obtain a more accurate expression of the forces between two bodies. This procedure also considers the relative orientation of the approaching surfaces.[8]
See also
References
- ↑ Derjaguin, B.V. (1934). "Untersuchungen über die Reibung und Adhäsion, IV. Theorie des Anhaftens kleiner Teilchen" [Analysis of friction and adhesion, IV. The theory of the adhesion of small particles]. Kolloid Z. (in German) 69 (2): 155–164. doi:10.1007/BF01433225.
- ↑ Russel, W.B.; Saville, D.A.; Schowalter, W.R. (1989). Colloidal Dispersions. Cambridge University Press. ISBN 978-0521426008.
- ↑ J. Israelachvili, Intermolecular and Surface Forces, Academic Press, London, 1992.
- ↑ Ducker, W. A.; Senden, T. J.; Pashley, R. M. (1991). "Direct measurement of colloidal forces using an atomic force microscope". Nature 353 (6341): 239. doi:10.1038/353239a0.
Butt, H. J. R. (1991). "Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope". Biophysical Journal 60 (6): 1438–1444. doi:10.1016/S0006-3495(91)82180-4. PMC 1260203. PMID 19431815. - ↑ Rentsch, S.; Pericet-Camara, R.; Papastavrou, G.; Borkovec, M. (2006). "Probing the validity of the Derjaguin approximation for heterogeneous colloidal particles". Physical Chemistry Chemical Physics 8 (21): 2531–2538. Bibcode:2006PCCP....8.2531R. doi:10.1039/B602145J. PMID 16721438.
- ↑ 6.0 6.1 White, L. R. (1983). "On the deryaguin approximation for the interaction of macrobodies". Journal of Colloid and Interface Science 95: 286–288. doi:10.1016/0021-9797(83)90103-0.
- ↑ Adamczyk, Z.; Weroński, P. (1999). "Application of the DLVO theory for particle deposition problems". Advances in Colloid and Interface Science 83: 137. doi:10.1016/S0001-8686(99)00009-3.
- ↑ Bhattacharjee, S.; Elimelech, M. (1997). "Surface Element Integration: A Novel Technique for Evaluation of DLVO Interaction between a Particle and a Flat Plate". Journal of Colloid and Interface Science 193 (2): 273–285. doi:10.1006/jcis.1997.5076. PMID 9344528.
Further reading
- Zypman, F.R. (2006). "Exact expressions for colloidal plane–particle interaction forces and energies with applications to atomic force microscopy". J. Phys.: Condens. Matter 8 (10): 2795. doi:10.1088/0953-8984/18/10/005.