Depsipeptide
A depsipeptide is a peptide in which one or more of the amide (-CONHR-) bonds are replaced by ester (COOR) bonds.[1]
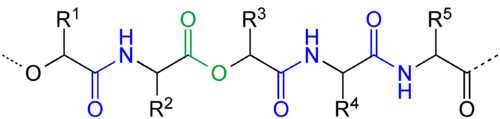
Depsipeptides have often been used in research to probe the importance of hydrogen bond networks in protein folding kinetics and thermodynamics. They are also found in nature as natural products. An infamous example is the L-Lys-D-Ala-D-Lac motif found in vancomycin resistant bacteria's cell wall building blocks. The amide to ester mutation disrupts its hydrogen bonding network with vancomycin, which is key to its antibiotic activity.
Medicine
Depsipeptide is also used to refer to romidepsin which is a member of the bicyclic peptide class of histone deacetylase inhibitors and was first isolated as a fermentation product from Chromobacterium violaceum by the Fujisawa Pharmaceutical Company.[2] It is being used in the treatment of some cancers, where it is thought to reactivate silenced genes.
The depsipeptide etamycin, a newly isolated natural product from a marine actinomycete, shows potent activity in vitro and in mice against MRSA.[3] It was first observed to have a positive role on gene expression in 1990. A clinical trial was conducted in 1996 for T-cell lymphoma.[4]
Another natural depsipeptide HDAC inhibitor is spiruchostatin A.[5]
Several depsipeptides have been found to inhibit HIV, including papuamide,[6] neamphamide A,[7] callipeltin A,[8] and mirabamides A-D.[9][10]
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1995) "depsipeptides".
- ↑ Yurek-George, Alexander; Cecil, Alexander Richard Liam; Mo, Alex Hon Kit; Wen, Shijun; Rogers, Helen; Habens, Fay; Maeda, Satoko; Yoshida, Minoru et al. (2007). "The First Biologically Active Synthetic Analogues of FK228, the Depsipeptide Histone Deacetylase Inhibitor". Journal of Medicinal Chemistry 50 (23): 5720–5726. doi:10.1021/jm0703800. PMID 17958342.
- ↑ Haste, Nina M; Perera, Varahenage R; Maloney, Katherine N; Tran, Dan N; Jensen, Paul; Fenical, William; Nizet, Victor; Hensler, Mary E (2010). "Activity of the streptogramin antibiotic etamycin against methicillin-resistant Staphylococcus aureus". J. Antibiotics 63 (5): 219. doi:10.1038/ja.2010.22.
- ↑ Depsipeptide (NSC 630176), National Cancer Institute
- ↑ "Total synthesis of Spiruchostatin A". Nippon Kagakkai Koen Yokoshu 86 (2): 1357. 2006.
- ↑ Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, de Silva ED, Lassota P, Allen TM, Van Soest R, Andersen RJ, Boyd MR. Papuamides A-D, HIV-Inhibitory and Cytotoxic Depsipeptides from the Sponges Theonella mirabilis and Theonella swinhoei Collected in Papua New Guinea. J. Am. Chem. Soc. 1999;121:5899–5909
- ↑ Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, Hess S, Pannell LK, Boyd MR, McMahon JB. Neamphamide A. A new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod. 2004;67(8):1407-11.
- ↑ Zampella A, D'Auria MV, Paloma LG, Casapullo A, Minale L, Debitus C, Henin Y. Callipeltin A, an Anti-HIV Cyclic Depsipeptide from the New Caledonian Lithistida Sponge Callipelta sp. J. Am. Chem. Soc. 1996;118:6202-9
- ↑ Plaza A, Gustchina E, Baker HL, Kelly M, Bewley CA. Mirabamides A-D. Depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J. Nat. Prod. 2007;70(11):1753-60
- ↑ Andjelic CD, Planelles V, Barrows LR. Characterizing the Anti-HIV Activity of Papuamide A. Mar Drugs. 2008;6(4):528-49