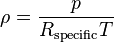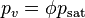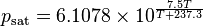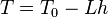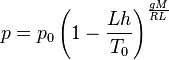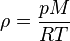Density of air
The density of air, ρ (Greek: rho) (air density), is the mass per unit volume of Earth's atmosphere, and is a useful value in aeronautics and other sciences. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in temperature or humidity. At sea level and at 15 °C, air has a density of approximately 1.225 kg/m3 (0.0023769 slugs/ft3, 0.001225 g/cm3) according to ISA (International Standard Atmosphere).
Temperature and pressure
The density of dry air can be calculated using the ideal gas law, expressed as a function of temperature and pressure:
where ρ is the air density, p is absolute pressure, Rspecific is the specific gas constant for dry air, and T is absolute temperature (K).
The specific gas constant for dry air is 287.058 J/(kg·K) in SI units, and 53.35 (ft·lbf)/(lbm·R) in United States customary and Imperial units. This quantity may vary slightly depending on the molecular composition of air at a particular location.
Therefore:
- At IUPAC standard temperature and pressure (0 °C and 100 kPa), dry air has a density of 1.2754 kg/m3.
- At 20 °C and 101.325 kPa, dry air has a density of 1.2041 kg/m3.
- At 70 °F and 14.696 psi, dry air has a density of 0.074887lbm/ft3.
The following table illustrates the air density–temperature relationship at 1 atm or 101.325 kPa:
| Temperature T in °C | Speed of sound c in m·s−1 | Density of air ρ in kg·m−3 | Acoustic impedance Z in N·s·m−3 |
|---|---|---|---|
| +35 | 351.88 | 1.1455 | 403.2 |
| +30 | 349.02 | 1.1644 | 406.5 |
| +25 | 346.13 | 1.1839 | 409.4 |
| +20 | 343.21 | 1.2041 | 413.3 |
| +15 | 340.27 | 1.2250 | 416.9 |
| +10 | 337.31 | 1.2466 | 420.5 |
| +5 | 334.32 | 1.2690 | 424.3 |
| 0 | 331.30 | 1.2922 | 428.0 |
| −5 | 328.25 | 1.3163 | 432.1 |
| −10 | 325.18 | 1.3413 | 436.1 |
| −15 | 322.07 | 1.3673 | 440.3 |
| −20 | 318.94 | 1.3943 | 444.6 |
| −25 | 315.77 | 1.4224 | 449.1 |
Water vapor
The addition of water vapor to air (making the air humid) reduces the density of the air, which may at first appear counter-intuitive.
This occurs because the molecular mass of water (18 g/mol) is less than the molecular mass of dry air (around 29 g/mol). For any gas, at a given temperature and pressure, the number of molecules present is constant for a particular volume (see Avogadro's Law). So when water molecules (vapor) are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing. Hence the mass per unit volume of the gas (its density) decreases.
The density of humid air may be calculated as a mixture of ideal gases. In this case, the partial pressure of water vapor is known as the vapor pressure. Using this method, error in the density calculation is less than 0.2% in the range of −10 °C to 50 °C. The density of humid air is found by:
where:
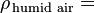 Density of the humid air (kg/m³)
Density of the humid air (kg/m³)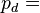 Partial pressure of dry air (Pa)
Partial pressure of dry air (Pa) Specific gas constant for dry air, 287.058 J/(kg·K)
Specific gas constant for dry air, 287.058 J/(kg·K)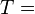 Temperature (K)
Temperature (K)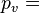 Pressure of water vapor (Pa)
Pressure of water vapor (Pa)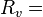 Specific gas constant for water vapor, 461.495 J/(kg·K)
Specific gas constant for water vapor, 461.495 J/(kg·K) Molar mass of dry air, 0.028964 (kg/mol)
Molar mass of dry air, 0.028964 (kg/mol)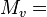 Molar mass of water vapor, 0.018016 (kg/mol)
Molar mass of water vapor, 0.018016 (kg/mol)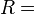 Universal gas constant, 8.314 J/(K·mol)
Universal gas constant, 8.314 J/(K·mol)
The vapor pressure of water may be calculated from the saturation vapor pressure and relative humidity. It is found by:
Where:
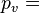 Vapor pressure of water
Vapor pressure of water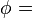 Relative humidity
Relative humidity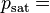 Saturation vapor pressure
Saturation vapor pressure
The saturation vapor pressure of water at any given temperature is the vapor pressure when relative humidity is 100%. One formula [2] used to find the saturation vapor pressure is:
where T is in degrees C. Note:
- This will give a result in hPa (100 Pa, equivalent to the older unit millibar, 1 mbar = 0.001 bar = 0.1 kPa)
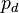 is found considering partial pressure, resulting in:
is found considering partial pressure, resulting in:
Where p simply denotes the observed absolute pressure.
Altitude
To calculate the density of air as a function of altitude, one requires additional parameters. They are listed below, along with their values according to the International Standard Atmosphere, using the universal gas constant instead of the specific one:
- sea level standard atmospheric pressure p0 = 101.325 kPa
- sea level standard temperature T0 = 288.15 K
- Earth-surface gravitational acceleration g = 9.80665 m/s2.
- temperature lapse rate L = 0.0065 K/m
- ideal (universal) gas constant R = 8.31447 J/(mol·K)
- molar mass of dry air M = 0.0289644 kg/mol
Temperature at altitude h meters above sea level is approximated by the following formula (only valid inside the troposphere):
The pressure at altitude h is given by:
Density can then be calculated according to a molar form of the ideal gas law:
where M is molar mass, R is the ideal gas constant, and T is absolute temperature. Note that p must be in Pa and not the kPa above.
See also
References
External links
- Conversions of density units ρ
- Air density and density altitude calculations
- Air Density Calculator
- Atmospheric Pressure Calculator