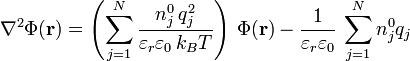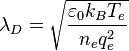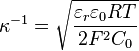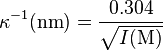Debye length
In plasmas and electrolytes the Debye length (also called Debye radius), named after the Dutch physicist and physical chemist Peter Debye, is the measure of a charge carrier's net electrostatic effect in solution, and how far those electrostatic effects persist. A Debye sphere is a volume whose radius is the Debye length, in which there is a sphere of influence, and outside of which charges are electrically screened. The notion of Debye length plays an important role in plasma physics, electrolytes and colloids (DLVO theory).
Physical origin
The Debye length arises naturally in the thermodynamic description of large systems of mobile charges. In a system of  different species of charges, the
different species of charges, the  -th species carries charge
-th species carries charge 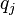 and has concentration
and has concentration 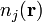 at position
at position  . According to the so-called "primitive model", these charges are distributed in a continuous medium that is characterized only by its relative static permittivity,
. According to the so-called "primitive model", these charges are distributed in a continuous medium that is characterized only by its relative static permittivity,  .
This distribution of charges within this medium gives rise to an electric potential
.
This distribution of charges within this medium gives rise to an electric potential 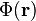 that satisfies Poisson's equation:
that satisfies Poisson's equation:
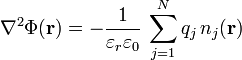 ,
,
where  is the electric constant.
is the electric constant.
The mobile charges not only establish 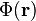 but also move in response to the associated Coulomb force,
but also move in response to the associated Coulomb force, 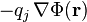 .
If we further assume the system to be in thermodynamic equilibrium with a heat bath at absolute temperature
.
If we further assume the system to be in thermodynamic equilibrium with a heat bath at absolute temperature  , then the
concentrations of discrete charges,
, then the
concentrations of discrete charges, 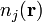 , may be considered to be
thermodynamic (ensemble) averages and the associated electric potential to be
a thermodynamic mean field.
With these assumptions, the concentration of the
, may be considered to be
thermodynamic (ensemble) averages and the associated electric potential to be
a thermodynamic mean field.
With these assumptions, the concentration of the  -th charge species is described
by the Boltzmann distribution,
-th charge species is described
by the Boltzmann distribution,
 ,
,
where  is Boltzmann's constant and where
is Boltzmann's constant and where 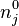 is the mean
concentration of charges of species
is the mean
concentration of charges of species  .
.
Identifying the instantaneous concentrations and potential in the Poisson equation with their mean-field counterparts in Boltzmann's distribution yields the Poisson-Boltzmann equation:
 .
.
Solutions to this nonlinear equation are known for some simple systems. Solutions for more general
systems may be obtained in the high-temperature (weak coupling) limit,  , by Taylor expanding the exponential:
, by Taylor expanding the exponential:
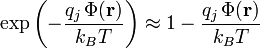 .
.
This approximation yields the linearized Poisson-Boltzmann equation
which also is known as the Debye-Hückel equation:[1][2][3][4][5] The second term on the right-hand side vanishes for systems that are electrically neutral. The term in parentheses has the units of an inverse length squared and by dimensional analysis leads to the definition of the characteristic length scale
that commonly is referred to as the Debye-Hückel length. As the only characteristic length scale in the Debye-Hückel equation, 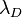 sets the scale for variations in the potential and in the concentrations of charged species. All charged species contribute to the Debye-Hückel length in the same way, regardless of the sign of their charges.
sets the scale for variations in the potential and in the concentrations of charged species. All charged species contribute to the Debye-Hückel length in the same way, regardless of the sign of their charges.
The Debye-Hückel length may be expressed in terms of the Bjerrum length 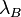 as
as
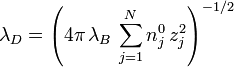 ,
,
where 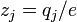 is the integer charge number that relates the charge on the
is the integer charge number that relates the charge on the  -th ionic
species to the elementary charge
-th ionic
species to the elementary charge  .
.
Typical values
In space plasmas where the electron density is relatively low, the Debye length may reach macroscopic values, such as in the magnetosphere, solar wind, interstellar medium and intergalactic medium (see table):
| Plasma | Density ne(m-3) |
Electron temperature T(K) |
Magnetic field B(T) |
Debye length λD(m) |
|---|---|---|---|---|
| Solar core | 1032 | 107 | -- | 10−11 |
| Tokamak | 1020 | 108 | 10 | 10−4 |
| Gas discharge | 1016 | 104 | -- | 10−4 |
| Ionosphere | 1012 | 103 | 10−5 | 10−3 |
| Magnetosphere | 107 | 107 | 10−8 | 102 |
| Solar wind | 106 | 105 | 10−9 | 10 |
| Interstellar medium | 105 | 104 | 10−10 | 10 |
| Intergalactic medium | 1 | 106 | -- | 105 |
| Source: Chapter 19: The Particle Kinetics of Plasma | ||||
Hannes Alfvén pointed out that: "In a low density plasma, localized space charge regions may build up large potential drops over distances of the order of some tens of the Debye lengths. Such regions have been called electric double layers. An electric double layer is the simplest space charge distribution that gives a potential drop in the layer and a vanishing electric field on each side of the layer. In the laboratory, double layers have been studied for half a century, but their importance in cosmic plasmas has not been generally recognized."[citation needed]
Debye length in a plasma
In a plasma, the background medium may be treated as the vacuum
(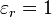 ), and the Debye length is
), and the Debye length is
where
- λD is the Debye length,
- ε0 is the permittivity of free space,
- kB is the Boltzmann constant,
- qe is the charge of an electron,
- Te and Ti are the temperatures of the electrons and ions, respectively,
- ne is the density of electrons,
- nij is the density of atomic species i, with positive ionic charge jqe
The ion term is often dropped, giving
although this is only valid when the mobility of ions is negligible compared to the process's timescale.[6]
Debye length in an electrolyte
In an electrolyte or a colloidal suspension, the Debye length[7] is usually denoted with symbol κ−1
where
- I is the ionic strength of the electrolyte, and here the unit should be mole/m3,
- ε0 is the permittivity of free space,
- εr is the dielectric constant,
- kB is the Boltzmann constant,
- T is the absolute temperature in kelvins,
- NA is the Avogadro number.
- e is the elementary charge,
or, for a symmetric monovalent electrolyte,
where
- R is the gas constant,
- F is the Faraday constant,
- C0 is the molar concentration of the electrolyte.
Alternatively,
where
-
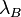 is the Bjerrum length of the medium.
is the Bjerrum length of the medium.
For water at room temperature, λB ≈ 0.7 nm.
At room temperature (25 °C), one can consider in water for 1:1 electrolytes the relation:[8]
where
- κ−1 is expressed in nanometers (nm)
- I is the ionic strength expressed in molar (M or mol/L)
Debye length in semiconductors
The Debye length has become increasingly significant in the modeling of solid state devices as improvements in lithographic technologies have enabled smaller geometries.[9][10][11]
The Debye length of semiconductors is given:
where
- εSi is the dielectric constant,
- kB is the Boltzmann's constant,
- T is the absolute temperature in kelvins,
- q is the elementary charge, and
- Nd is the density of dopants (either donors or acceptors).
When doping profiles exceed the Debye length, majority carriers no longer behave according to the distribution of the dopants. Instead, a measure of the profile of the doping gradients provides an “effective” profile that better matches the profile of the majority carrier density.
In the context of solids, the Debye length is also called the Thomas–Fermi screening length.
See also
Debye-Falkenhagen effect
References
- ↑ Kirby BJ. Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices.
- ↑ Li D (2004). Electrokinetics in Microfluidics.
- ↑ PC Clemmow & JP Dougherty (1969). Electrodynamics of particles and plasmas. Redwood City CA: Addison-Wesley. pp. §7.6.7, p. 236 ff. ISBN 0-201-47986-9.
- ↑ RA Robinson &RH Stokes (2002). Electrolyte solutions. Mineola NY: Dover Publications. p. 76. ISBN 0-486-42225-9.
- ↑ See DC Brydges & Ph A Martin Coulomb Systems at Low Density: A Review
- ↑ I. H. Hutchchinson - Principles of plasma diagnostics; ISBN 0-521-38583-0
- ↑ Russel, W.B., Saville, D.A. and Schowalter, W.R. Colloidal Dispersions, Cambridge University Press, 1989
- ↑ Israelachvili, J., Intermolecular and Surface Forces, Academic Press Inc., 1985, ISBN 0-12-375181-0
- ↑ Stern, Eric; Robin Wagner, Fred J. Sigworth, Ronald Breaker, Tarek M. Fahmy, Mark A. Reed (2007-11-01). "Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors". Nano Letters 7 (11): 3405–3409. Bibcode:2007NanoL...7.3405S. doi:10.1021/nl071792z.
- ↑ Guo, Lingjie; Effendi Leobandung, Stephen Y. Chou (1997). "A room-temperature silicon single-electron metal–oxide–semiconductor memory with nanoscale floating-gate and ultranarrow channel". Applied Physics Letters 70 (7): 850. Bibcode:1997ApPhL..70..850G. doi:10.1063/1.118236. ISSN 0003-6951. Retrieved 2010-10-25.
- ↑ Tiwari, Sandip; Farhan Rana, Kevin Chan, Leathen Shi, Hussein Hanafi (1996). "Single charge and confinement effects in nano-crystal memories". Applied Physics Letters 69 (9): 1232. Bibcode:1996ApPhL..69.1232T. doi:10.1063/1.117421. ISSN 0003-6951. Retrieved 2010-10-25.
Further reading
- Goldston & Rutherford (1997). Introduction to Plasma Physics. Institute of Physics Publishing, Philadelphia.
- Lyklema (1993). Fundamentals of Interface and Colloid Science. Academic Press, NY.