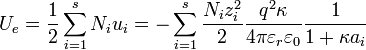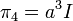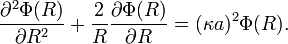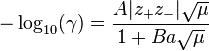Debye–Hückel equation
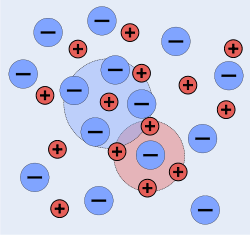
The Debye–Hückel equation and Debye–Hückel limiting law were derived by Peter Debye and Erich Hückel, who developed a theory with which to calculate activity coefficients of electrolyte solutions. Activities, rather than concentrations, are needed in many chemical calculations because solutions that contain ionic solutes do not behave ideally even at very low concentrations. The activity is proportional to the concentration by a factor known as the activity coefficient 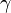 , and takes into account the interaction energy of ions in the solution.
, and takes into account the interaction energy of ions in the solution.
Debye-Hückel limiting law
- For the principles used to derive this equation see Debye-Hückel theory
In order to calculate the activity,  , of an ion, C, in a solution, one must know the concentration and the activity coefficient:
, of an ion, C, in a solution, one must know the concentration and the activity coefficient:
where
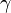 is the activity coefficient of C
is the activity coefficient of C ![\ [C^{{\ominus }}]\,](/2014-wikipedia_en_all_02_2014/I/media/e/0/7/0/e07049418a3fef8d070e01d17b0cb372.png) is the concentration of the chosen standard state, e.g. 1 mol/kg if we work in molality.
is the concentration of the chosen standard state, e.g. 1 mol/kg if we work in molality.![[C]](/2014-wikipedia_en_all_02_2014/I/media/9/c/5/5/9c550142b2e58d791444e540ee6ed5e6.png) is a dimensionless measure of the concentration of C
is a dimensionless measure of the concentration of C
The Debye-Hückel limiting law enables one to determine the activity coefficient of an ion in a dilute solution of known ionic strength. The equation is:section 2.5.2
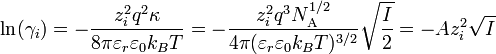
 is the charge number of ion species i
is the charge number of ion species i is the elementary charge
is the elementary charge is the inverse of the Debye screening length, defined below
is the inverse of the Debye screening length, defined below is the relative permittivity of the solvent
is the relative permittivity of the solvent is the permittivity of free space
is the permittivity of free space is Boltzmann's constant
is Boltzmann's constant is the temperature of the solution
is the temperature of the solution is Avogadro's number
is Avogadro's number is the ionic strength of the solution, defined below
is the ionic strength of the solution, defined below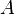 is a constant that depends on the solvent. If
is a constant that depends on the solvent. If  is expressed in terms of molality, instead of molarity (as in the equation above and in the rest of this article), then an experimental value for
is expressed in terms of molality, instead of molarity (as in the equation above and in the rest of this article), then an experimental value for 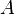 is
is  . In fact it is common to use a base-10 logarithm, in which case we factor
. In fact it is common to use a base-10 logarithm, in which case we factor  , so A is
, so A is  .
.
It is important to note that because the ions in the solution act together, the activity coefficient obtained from this equation is actually a mean activity coefficient.
Summary of Debye & Hückel's first paper on the theory of dilute electrolytes
The English title of the paper is called "On the Theory of Electrolytes. I. Freezing Point Depression and Related Phenomena." It was originally published in volume 24 of a German-language journal, called Physikalische Zeitschrift, in 1923. An English translation[1]:217-63of the paper is included in a book of collected papers presented to Debye by "his pupils, friends, and the publishers on the occasion of his seventieth birthday on March 24, 1954."[1]:xv The paper deals with the calculation of properties of electrolyte solutions that are not under the influence of net electric fields, thus it deals with electrostatics.
In the same year they first published this paper, Debye and Hückel, hereinafter D&H, also released a paper that covered their initial characterization of solutions under the influence of electric fields called "On the Theory of Electrolytes. II. Limiting Law for Electric Conductivity," but that subsequent paper is not (yet) covered here.
In the following summary (as yet incomplete and unchecked), modern notation and terminology are used, from both chemistry and mathematics, in order to prevent confusion. Also, with a few exceptions to improve clarity, the subsections in this summary are (very) condensed versions of the same subsections of the original paper.
Introduction
D&H note that the Guldberg-Waage formula for electrolyte species in chemical reaction equilibrium in classical form is[1]:221
 is a notation for multiplication
is a notation for multiplication is a dummy variable indicating the species
is a dummy variable indicating the species is the number of species participating in the reaction
is the number of species participating in the reaction is the mole fraction of species
is the mole fraction of species 
 is the stoichiometric coefficient of species
is the stoichiometric coefficient of species 
- K is the equilibrium constant.
D&H say that, due to the "mutual electrostatic forces between the ions," it is necessary to modify the Guldberg-Waage equation by replacing  with
with 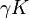 , where
, where  is an overall activity coefficient, not a "special" activity coefficient (a separate activity coefficient associated for each species)—which is what is used in modern chemistry as of 2007.
is an overall activity coefficient, not a "special" activity coefficient (a separate activity coefficient associated for each species)—which is what is used in modern chemistry as of 2007.
The relationship between  and the special activity coefficients,
and the special activity coefficients,  is[1]:248
is[1]:248
Fundamentals
D&H use the Helmholtz and Gibbs free entropies,  and
and  , to express the effect of electrostatic forces in an electrolyte on its thermodynamic state. Specifically, they split most of the thermodynamic potentials into classical and electrostatic terms.
, to express the effect of electrostatic forces in an electrolyte on its thermodynamic state. Specifically, they split most of the thermodynamic potentials into classical and electrostatic terms.
 is Helmholtz free entropy
is Helmholtz free entropy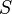 is entropy
is entropy is internal energy
is internal energy is temperature
is temperature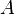 is Helmholtz free energy
is Helmholtz free energy
D&H give the total differential of  as[1]:222
as[1]:222
By the definition of the total differential, this means that
 and
and
 ,
,
which are useful further on.
As stated previously, the internal energy is divided into two parts,[1]:222
 indicates the classical part
indicates the classical part indicates the electric part
indicates the electric part
Similarly, the Helmholtz free entropy is also divided into two parts,
D&H state, without giving the logic, that[1]:222
It would seem that, without some justification,
 .
.
Without mentioning it specifically, D&H later give what might be the required (above) justification while arguing that 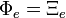 , an assumption that the solvent is incompressible.
, an assumption that the solvent is incompressible.
The definition of the Gibbs free entropy,  , is[1]:222-3
, is[1]:222-3
 .
.
D&H give the total differential of  as[1]:222
as[1]:222
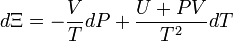 .
.
At this point D&H note that, for water containing 1 mole per liter of potassium chloride (nominal pressure and temperature aren't given), the electric pressure,  , amounts to 20 atmospheres. Furthermore, they note that this level of pressure gives a relative volume change of 0.001. Therefore, they neglect change in volume of water due to electric pressure, writing[1]:223
, amounts to 20 atmospheres. Furthermore, they note that this level of pressure gives a relative volume change of 0.001. Therefore, they neglect change in volume of water due to electric pressure, writing[1]:223
and put
 .
.
D&H say that, according to Planck, the classical part of the Gibbs free entropy is[1]:223
 .
.
 is a species
is a species is the number of different particle types in solution
is the number of different particle types in solution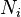 is the number of particles of species i
is the number of particles of species i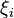 is the particle specific Gibbs free entropy of species i
is the particle specific Gibbs free entropy of species i is Boltzmann's constant
is Boltzmann's constant is the mole fraction of species i
is the mole fraction of species i
Species zero is the solvent. The definition of 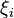 is as follows, where lower case letters indicate the particle specific versions of the corresponding extensive properties:[1]:223
is as follows, where lower case letters indicate the particle specific versions of the corresponding extensive properties:[1]:223
 .
.
D&H don't say so, but the functional form for 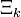 may be derived from the functional dependence of the chemical potential of a component of an ideal mixture upon its mole fraction.[2]
may be derived from the functional dependence of the chemical potential of a component of an ideal mixture upon its mole fraction.[2]
D&H note that the internal energy,  , of a solution is lowered by the electrical interaction of its ions, but that this effect can't be determined by using the crystallographic approximation for distances between dissimilar atoms (the cube root of the ratio of total volume to the number of particles in the volume). This is because there is more thermal motion in a liquid solution than in a crystal. The thermal motion tends to smear out the natural lattice that would otherwise be constructed by the ions. Instead, D&H introduce the concept of an ionic cloud. Like the crystal lattice, each ion still attempts to surround itself with oppositely charged ions, but in a more free-form manner; at small distances away from positive ions, one is more likely to find negative ions and vice versa.[1]:225
, of a solution is lowered by the electrical interaction of its ions, but that this effect can't be determined by using the crystallographic approximation for distances between dissimilar atoms (the cube root of the ratio of total volume to the number of particles in the volume). This is because there is more thermal motion in a liquid solution than in a crystal. The thermal motion tends to smear out the natural lattice that would otherwise be constructed by the ions. Instead, D&H introduce the concept of an ionic cloud. Like the crystal lattice, each ion still attempts to surround itself with oppositely charged ions, but in a more free-form manner; at small distances away from positive ions, one is more likely to find negative ions and vice versa.[1]:225
The Potential Energy of an Arbitrary Ion Solution
Electroneutrality of a solution requires that[1]:233
 .
.
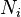 is the total number of ions of species i in the solution
is the total number of ions of species i in the solution is the charge number of species i.
is the charge number of species i.
To bring an ion of species i, initially far away, to a point  within the ion cloud requires interaction energy in the amount of
within the ion cloud requires interaction energy in the amount of  , where
, where  is the elementary charge and
is the elementary charge and  is the value of the scalar electric potential field at
is the value of the scalar electric potential field at  . If electric forces were the only factor in play, the minimum energy configuration of all the ions would be achieved in a close-packed lattice configuration. However, the ions are in thermal equilibrium with each other and they are relatively free to move. Thus they obey Boltzmann statistics and form a Boltzmann distribution. All species' number densities,
. If electric forces were the only factor in play, the minimum energy configuration of all the ions would be achieved in a close-packed lattice configuration. However, the ions are in thermal equilibrium with each other and they are relatively free to move. Thus they obey Boltzmann statistics and form a Boltzmann distribution. All species' number densities,  , are altered from their bulk (overall average) values,
, are altered from their bulk (overall average) values, 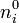 , by the corresponding Boltzmann factor,
, by the corresponding Boltzmann factor, 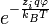 , where
, where  is the Boltzmann constant and
is the Boltzmann constant and  is the temperature (http://www.pma.caltech.edu/Courses/ph136/yr2006/text.html, section 19.3). Thus,[1]:233
is the temperature (http://www.pma.caltech.edu/Courses/ph136/yr2006/text.html, section 19.3). Thus,[1]:233

- V is the volume of the solution
at every point in the cloud. Note that in the infinite temperature limit, all ions are distributed uniformly, with no regard for their electrostatic interactions.[1]:227
The charge density is related to the number density:[1]:233
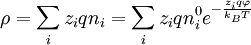 .
.
When combining this result for the charge density with the Poisson equation from electrostatics, a form of the Poisson-Boltzmann equation results:[1]:233
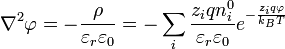 .
.
This equation is difficult to solve and does not follow the principle of linear superposition for the relationship between the number of charges and the strength of the potential field. However, for sufficiently low concentrations of ions, a first order Taylor series approximation for the exponential function may be used (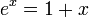 for
for  ) to create a linear differential equation (Hamann, Hamnett, and Vielstich. Electrochemistry. Wiley-VCH. section 2.4.2). D&H say that this approximation holds at large distances between ions,[1]:227 which is the same as saying that the concentration is low. Lastly, they claim without proof that the addition of more terms in the expansion has little effect on the final solution.[1]:227 Thus,
) to create a linear differential equation (Hamann, Hamnett, and Vielstich. Electrochemistry. Wiley-VCH. section 2.4.2). D&H say that this approximation holds at large distances between ions,[1]:227 which is the same as saying that the concentration is low. Lastly, they claim without proof that the addition of more terms in the expansion has little effect on the final solution.[1]:227 Thus,
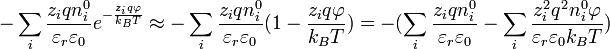 .
.
The Poisson-Boltzmann equation is transformed to[1]:233
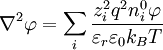 ,
,
because the first summation is zero due to electroneutrality.[1]:234
Factor out the scalar potential and assign the leftovers, which are constant, to  . Also, let
. Also, let  be the ionic strength of the solution:[1]:234
be the ionic strength of the solution:[1]:234
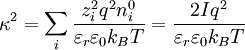 ,
,
 .
.
So, the fundamental equation is reduced to a form of the Helmholtz equation:(http://guava.physics.uiuc.edu/~nigel/courses/569/Essays_2004/files/lu.pdf section 3.1)
 .
.
Today, 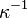 is called the Debye screening length. D&H recognize the importance of the parameter in their paper and characterize it as a measure of the thickness of the ion atmosphere, which is an electrical double layer of the Guoy-Chapman type.[1]:229
is called the Debye screening length. D&H recognize the importance of the parameter in their paper and characterize it as a measure of the thickness of the ion atmosphere, which is an electrical double layer of the Guoy-Chapman type.[1]:229
The equation may be expressed in spherical coordinates by taking  at some arbitrary ion (http://hyperphysics.phy-astr.gsu.edu/hbase/electric/laplace.html):[1]:229
at some arbitrary ion (http://hyperphysics.phy-astr.gsu.edu/hbase/electric/laplace.html):[1]:229
 .
.
The equation has the following general solution; keep in mind that  is a positive constant:[1]:229
is a positive constant:[1]:229
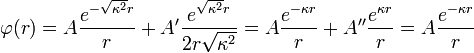 .
.
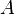 ,
, 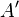 , and
, and 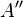 are undetermined constants
are undetermined constants
The electric potential is zero at infinity by definition, so 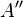 must be zero.[1]:229
must be zero.[1]:229
In the next step, D&H assume that there is a certain radius,  , beyond which no ions in the atmosphere may approach the (charge) center of the singled out ion. This radius may be due to the physical size of the ion itself, the sizes of the ions in the cloud, and any water molecules that surround the ions. Mathematically, they treat the singled out ion as a point charge to which one may not approach within the radius
, beyond which no ions in the atmosphere may approach the (charge) center of the singled out ion. This radius may be due to the physical size of the ion itself, the sizes of the ions in the cloud, and any water molecules that surround the ions. Mathematically, they treat the singled out ion as a point charge to which one may not approach within the radius  .[1]:231
.[1]:231
The potential of a point charge by itself is:
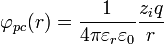 .
.
D&H say that the total potential inside the sphere is[1]:232
 ,
,
where 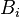 is a constant that represents the potential added by the ionic atmosphere. No justification for
is a constant that represents the potential added by the ionic atmosphere. No justification for 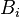 being a constant is given. However, one can see that this is the case by considering that any spherical static charge distribution is subject to the mathematics of the shell theorem. The shell theorem says that no force is exerted on charged particles inside a sphere (of arbitrary charge) (http://hyperphysics.phy-astr.gsu.edu/hbase/electric/potsph.html). Since the ion atmosphere is assumed to be (time-averaged) spherically symmetric, with charge varying as a function of radius
being a constant is given. However, one can see that this is the case by considering that any spherical static charge distribution is subject to the mathematics of the shell theorem. The shell theorem says that no force is exerted on charged particles inside a sphere (of arbitrary charge) (http://hyperphysics.phy-astr.gsu.edu/hbase/electric/potsph.html). Since the ion atmosphere is assumed to be (time-averaged) spherically symmetric, with charge varying as a function of radius  , it may be represented as an infinite series of concentric charge shells. Therefore, inside the radius
, it may be represented as an infinite series of concentric charge shells. Therefore, inside the radius  , the ion atmosphere exerts no force. If the force is zero, then the potential is a constant (by definition).
, the ion atmosphere exerts no force. If the force is zero, then the potential is a constant (by definition).
In a combination of the continuously distributed model which gave the Poisson-Boltzmann equation and the model of the point charge, it is assumed that at the radius  , there is a continuity of
, there is a continuity of  and its first derivative. Thus,[1]:232
and its first derivative. Thus,[1]:232
 ,
, ,
, , and
, and .
.
By the definition of electric potential energy, the potential energy associated with the singled out ion in the ion atmosphere is[1]:230 & 232
Notice that this only requires knowledge of the charge of the singled out ion and the potential of all the other ions.
To calculate the potential energy of the entire electrolyte solution, one must use the multiple charge generalization for electric potential energy.[1]:230 & 232
The Additional Electric Term to the Thermodynamic Potential
Nondimensionalization
This section was created without reference to the original paper and there are some errors in it (for instance, the ionic strength is off by a factor of two). Once these are rectified, this section should probably be moved to the nondimensionalization article and then be linked from here, since the nondimensional version of the Poisson-Boltzmann equation isn't necessary to understand the D&H theory.
The differential equation is ready for solution (as stated above, the equation only holds for low concentrations):
Using the Buckingham π theorem on this problem results in the following dimensionless groups:
 is called the reduced scalar electric potential field.
is called the reduced scalar electric potential field.  is called the reduced radius. The existing groups may be recombined to form two other dimensionless groups for substitution into the differential equation. The first is what could be called the square of the reduced inverse screening length,
is called the reduced radius. The existing groups may be recombined to form two other dimensionless groups for substitution into the differential equation. The first is what could be called the square of the reduced inverse screening length, 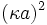 . The second could be called the reduced central ion charge,
. The second could be called the reduced central ion charge,  (with a capital Z). Note that, though
(with a capital Z). Note that, though  is already dimensionless, without the substitution given below, the differential equation would still be dimensional.
is already dimensionless, without the substitution given below, the differential equation would still be dimensional.
To obtain the nondimensionalized differential equation and initial conditions, use the  groups to eliminate
groups to eliminate  in favor of
in favor of 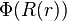 , then eliminate
, then eliminate  in favor of
in favor of  while carrying out the chain rule and substituting
while carrying out the chain rule and substituting  , then eliminate
, then eliminate  in favor of
in favor of  (no chain rule needed), then eliminate
(no chain rule needed), then eliminate  in favor of
in favor of 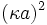 , then eliminate
, then eliminate  in favor of
in favor of  . The resulting equations are as follows:
. The resulting equations are as follows:
For table salt in 0.01 M solution at 25°C, a typical value of 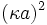 is 0.0005636, while a typical value of
is 0.0005636, while a typical value of  is 7.017, highlighting the fact that, in low concentrations,
is 7.017, highlighting the fact that, in low concentrations, 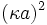 is a target for a zero order of magnitude approximation such as perturbation analysis. Unfortunately, because of the boundary condition at infinity, regular perturbation does not work. The same boundary condition prevents us from finding the exact solution to the equations. Singular perturbation may work, however.
is a target for a zero order of magnitude approximation such as perturbation analysis. Unfortunately, because of the boundary condition at infinity, regular perturbation does not work. The same boundary condition prevents us from finding the exact solution to the equations. Singular perturbation may work, however.
Experimental verification of the theory
To verify the validity of the Debye-Hückel theory, many experimental ways have been tried, measuring the activity coefficients: the problem is that we need to go towards very high dilutions. Typical examples are: measurements of vapour pressure, freezing point, osmotic pressure (indirect methods) and measurement of electric potential in cells (direct method). Going towards high dilutions goods results have been found using liquid membrane cells, it has been possible to investigate aqueous media 10−4 M and it has been found that for 1:1 electrolytes (as NaCl or KCl) the Debye–Hückel equation is totally correct, but for 2:2 or 3:2 electrolytes it is possible to find negative deviation from the Debye–Hückel limit law: this strange behavior can be observed only in the very dilute area, and in more concentrate regions the deviation becomes positive. It is possible that Debye–Hückel equation is not able to foresee this behavior because of the linearization of the Poisson-Boltzmann equation, or maybe not: studies about this have been started only during the last years of the 20th century because before it wasn’t possible to investigate the 10−4 M region, so it is possible that during the next years new theories will be born.
Extensions of the theory
Warning: The notation in this section is (currently) different than in the rest of the article.
A number of approaches have been proposed to extend the validity of the law to concentration ranges as commonly encountered in chemistry
One such Extended Debye-Hückel Equation is given by:
where 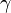 as its common logarithm is the activity coefficient,
as its common logarithm is the activity coefficient,  is the integer charge of the ion (1 for H+, 2 for Mg2+ etc.),
is the integer charge of the ion (1 for H+, 2 for Mg2+ etc.),  is the ionic strength of the aqueous solution, and
is the ionic strength of the aqueous solution, and  is the size or effective diameter of the ion in angstrom. The effective hydrated radius of the ion, a is the radius of the ion and its closely bound water molecules. Large ions and less highly charged ions bind water less tightly and have smaller hydrated radii than smaller, more highly charged ions. Typical values are 3Å for ions such as H+,Cl-,CN-, and HCOO-. The effective diameter for the hydronium ion is 9Å.
is the size or effective diameter of the ion in angstrom. The effective hydrated radius of the ion, a is the radius of the ion and its closely bound water molecules. Large ions and less highly charged ions bind water less tightly and have smaller hydrated radii than smaller, more highly charged ions. Typical values are 3Å for ions such as H+,Cl-,CN-, and HCOO-. The effective diameter for the hydronium ion is 9Å.  and
and  are constants with values of respectively 0.5085 and 0.3281 at 25°C in water .
are constants with values of respectively 0.5085 and 0.3281 at 25°C in water .
The Extended Debye-Hückel Equation provides accurate results for μ ≤ 0.1. For solutions of greater ionic strengths, the Pitzer equations should be used. In these solutions the activity coefficient may actually increase with ionic strength.

The Debye-Hückel Equation cannot be used in the solutions of surfactants where the presence of micelles influences on the electrochemical properties of the system (even rough judgement overestimates γ for ~50%).
See also
- Strong electrolyte
- Weak electrolyte
- Ionic atmosphere
- Debye–Hückel theory
Notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 The Collected Papers of Peter J. W. Debye. New York, New York: Interscience Publishers, Inc. 1954.
- ↑ Salzman, William R. (2001-08-21). "Mixtures; Partial Molar Quantities; Ideal Solutions". Chemical Thermodynamics. University of Arizona. Archived from the original on 2007-06-07. Retrieved 2007-10-23.
References
- P. Debye and E. Hückel (1923). "Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen" [The theory of electrolytes. I. Lowering of freezing point and related phenomena] (PDF). Physikalische Zeitschrift 24: 185–206.
- ^ Hamann, Hamnett, and Vielstich (1998). Electrochemistry. Weinheim: Wiley-VCH Verlag GmbH. ISBN 3-527-29096-6.
- ^ Harris, Daniel C. (2003). Quantitative Chemical Analysis (6th edition ed.). W. H. Freeman & Company. ISBN 0-7167-4464-3.
- ^ Skoog, Douglas A. Fundamentals of Analytical Chemistry. ISBN 0-534-41796-5.
- F. Malatesta, R. Zamboni. Activity and osmotic coefficents from the EMF of liquid membrane cells, VI - ZnSO4, MgSO4, CaSO4 and SrSO4 in water at 25 C. Journal of Solution Chemistry 1997, 26, 791-815
External links
- For easy calculation of activity coefficients in (non-micellar) solutions, check out the IUPAC open project Aq-solutions (freeware).
- Gold Book definition
![\ a_{C}=\gamma {\frac {[C]}{[C^{{\ominus }}]}}\,](/2014-wikipedia_en_all_02_2014/I/media/2/4/3/8/2438c5ba3f92afad99387dd569673d05.png)

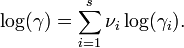

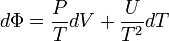
 is
is 
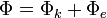
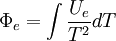
 is
is