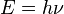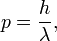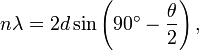Davisson–Germer experiment
The Davisson–Germer experiment was a physics experiment conducted by American physicists Clinton Davisson and Lester Germer in 1927,[1] which confirmed the de Broglie hypothesis. This hypothesis advanced by Louis de Broglie in 1924 says that particles of matter such as electrons have wave like properties. The experiment not only played a major role in verifying the de Broglie hypothesis and demonstrated the wave-particle duality, but also was an important historical development in the establishment of quantum mechanics and of the Schrödinger equation.
History and Overview
According to Maxwell's equations in the late 19th century, light was thought to consist of waves of electromagnetic fields and matter consist of localized particles. However this was challenged in Albert Einstein’s 1905 paper on the photoelectric effect, which described light as discrete and localized quanta of energy (now called photons), and won him the Nobel Prize in Physics in 1921. In 1927 Louis de Broglie presented his thesis concerning the wave-particle duality theory, which proposed the idea that all matter displays the wave-particle duality of photons.[2] According to de Broglie for all matter and for radiation alike, the energy E of the particle was related to the frequency of its associated wave ν by the Planck relation:
And that the momentum of the particle p was related to its wavelength by what is now known as the de Broglie relation:
where h is Planck's constant.
An important contribution to the Davisson–Germer experiment was made by Walter M. Elsasser in Göttingen in the 1920s, who remarked that the wave-like nature of matter might be investigated by electron scattering experiments on crystalline solids, just as the wave-like nature of X-rays had been confirmed through X-ray scattering experiments on crystalline solids.[2][3]
This suggestion of Elsasser was then communicated by his senior colleague (and later Nobel Prize recipient) Max Born to physicists in England. When the Davisson and Germer experiment was performed, the results of the experiment were explained by Elsasser's proposition. However the initial intention of the Davisson and Germer experiment was not to confirm the de Broglie hypothesis, but rather to study the surface of nickel.

In 1927 at Bell Labs, Clinton Davisson and Lester Germer fired slow moving electrons at a crystalline nickel target. The angular dependence of the reflected electron intensity was measured and was determined to have the same diffraction pattern as those predicted by Bragg for X-rays. This experiment was independently replicated by George Paget Thomson, and Davisson and Thomson shared the Nobel Prize in Physics in 1937.[2][4] The Davisson – Germer experiment confirmed the de Broglie hypothesis that matter has wave-like behavior. This, in combination with the Compton effect discovered by Arthur Compton (who won the Nobel Prize for Physics in 1927),[5] established the wave–particle duality hypothesis which was a fundamental step in quantum theory.
Experiment

Davisson and Germer's actual objective was to study the surface of a piece of nickel by directing a beam of electrons at the surface and observing how many electrons bounced off at various angles. They expected that for electrons even the smoothest crystal surface would be too rough and so the electron beam would experience diffuse reflection.[6]
The experiment consisted of firing an electron beam from an electron gun directed to a piece of nickel crystal at normal incidence (i.e. perpendicular to the surface of the crystal). The experiment included an electron gun consisting of a heated filament that released thermally excited electrons, which were then accelerated through a potential difference giving them a certain amount of kinetic energy towards the nickel crystal. To avoid collisions of the electrons with other molecules on their way towards the surface, the experiment was conducted in a vacuum chamber. To measure the number of electrons that were scattered at different angles, an electron detector that could be moved on an arc path about the crystal was used. The detector was designed to accept only elastically scattered electrons.
During the experiment an accident occurred and air entered the chamber, producing an oxide film on the nickel surface. To remove the oxide, Davisson and Germer heated the specimen in a high temperature oven, not knowing that this affected the formerly polycrystalline structure of the nickel to form large single crystal areas with crystal planes continuous over the width of the electron beam.[6]
When they started the experiment again and the electrons hit the surface, they were scattered by atoms which originated from crystal planes inside the nickel crystal. As Max von Laue proved in 1912 the crystal structure serves as a type of three dimensional diffraction grating. The angles of maximum reflection are given by Bragg's condition for constructive interference from an array, Bragg's law
for n = 1, θ = 50°, and for the spacing of the crystalline planes of nickel (d = 0.091 nm) obtained from previous X-ray scattering experiments on crystalline nickel.[2]
By varying the applied voltage to the electron gun, the maximum intensity of electrons diffracted by the atomic surface was found at different angles. The highest intensity was observed at an angle θ = 50° with a voltage of 54 V, giving the electrons a kinetic energy of 54 eV.[2]
According to the de Broglie relation and Bragg's law, a beam of 54 eV had a wavelength of 0.167 nm. The experimental outcome was 0.165 nm via the grating equation, which closely matched the predictions.
Davisson and Germer's accidental discovery of the diffraction of electrons was the first direct evidence confirming de Broglie's hypothesis that particles can have wave properties as well.
References
- ↑ Davisson, C.J. (January 1928). "The Diffraction of Electrons by a Crystal of Nickel". Bell System Tech. J. (USA: American Tel. & Tel.) 7 (1): 90–105. Retrieved December 5, 2012.
- ↑ 2.0 2.1 2.2 2.3 2.4 R. Eisberg, R. Resnick (1985). "Chapter 3 – de Broglie's Postulate—Wavelike Properties of Particles". Quantum Physics: of Atoms, Molecules, Solids, Nuclei, and Particles (2nd ed.). John Wiley & Sons. ISBN 0-471-87373-X.
- ↑ H. Rubin (1995). "Walter M. Elsasser". Biographical Memoirs 68. National Academy Press. ISBN 0-309-05239-4.
- ↑ The Nobel Foundation (Clinton Joseph Davisson and George Paget Thomson) (1937). "Clinton Joseph Davisson and George Paget Thomson for their experimental discovery of the diffraction of electrons by crystals". The Nobel Foundation 1937.
- ↑ The Nobel Foundation (Arthur Holly Compton and Charles Thomson Rees Wilson) (1937). "Arthur Holly Compton for his discovery of the effect named after him and Charles Thomson Rees Wilson for his method of making the paths of electrically charged particles visible by condensation of vapour". The Nobel Foundation 1927.
- ↑ 6.0 6.1 Hugh D. Young, Roger A. Freedman: University Physics, Ed. 11. Pearson Education, Addison Wesley, San Francisco 2004, 0-321-20469-7, S. 1493-1494.
External links
- R. Nave. "Davisson–Germer Experiment". HyperPhysics. Georgia State University, Physics Departement.