Cyclotriveratrylene
| Cyclotriveratrylene | ||
|---|---|---|
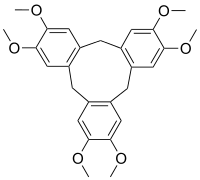 | ||
| IUPAC name 2,3,7,8,12,13-Hexamethoxy-10,15-dihydro-5H-tribenzo[a,d,g][9]annulene | ||
| Identifiers | ||
| PubChem | 273730 | |
| ChemSpider | 240862 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C27H30O6 | |
| Molar mass | 450.52 g mol−1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Cyclotriveratrylene (CTV) is a macrocycle and used in host-guest chemistry as a molecular host.[1] The compound can be synthesised from veratrole alcohol by addition of a suitable acid which can be perchloric acid in methanol or formic acid or sulfuric acid in acetic acid.
An alternative is synthesis from veratrole and formaldehyde.
The ether groups are situated at the upper rim. CTV has a bowl shaped conformation just like a crown ether. The compound is related to calixarenes.
CTV derivates are known to bind fullerenes and the use of CTV's in the separation of mixtures of fullerenes has been demonstrated. They have also been used in the solubilizing and immobilizing of fullerene compounds. CTV derivatives are also known to form liquid crystals and they are used as structural units for polymer networks (gels) and polymers. With suitable metal complexes CTV's can form metallo-supramolecular oligomers or polymers.
When two CTV units are connected though molecular spacers a molecular box is formed called a cryptophane. Cryptophanes are of interest in molecular encapsulation.
History
CTV was first synthesised by Gertrude Robinson (the first wife of Sir Robert Robinson) in 1915 [2] but misdiagnosed as the dimer. In 1965 Lindsey [3] identified the correct structure and coined cyclotriveratrylene as the name for the compound.[4]
References
- ↑ Hardie, M. (2010). "Recent advances in the chemistry of cyclotriveratrylene". Chemical Society reviews 39 (2): 516–527. doi:10.1039/b821019p. PMID 20111776.
- ↑ Robinson, G. M. (1915). "XXX.?A reaction of homopiperonyl and of homoveratryl alcohols". Journal of the Chemical Society, Transactions 107: 267–276. doi:10.1039/CT9150700267.
- ↑ Lindsey, A. S. (1965). "316. The structure of cyclotriveratrylene (10,15-dihydro-2,3,7,8,12,13-hexamethoxy-5H-tribenzo[a,d,g]cyclononene) and related compounds". Journal of the Chemical Society (Resumed): 1685–1692. doi:10.1039/JR9650001685.
- ↑ Supramolecular Chemistry Jonathan W. Steed, Jerry L. Atwood
