Cyclohexenone
| Cyclohexenone | |
|---|---|
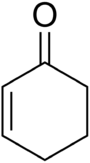 | |
| IUPAC name 1-Cyclohex-2-enone | |
| Identifiers | |
| CAS number | 930-68-7 |
| PubChem | 13594 |
| ChemSpider | 13005 |
| KEGG | C02395 |
| ChEBI | CHEBI:15977 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H8O |
| Molar mass | 96.13 g mol−1 |
| Appearance | Clear colorless liquid |
| Density | 0.993 g/mL [1] |
| Melting point | −53 °C; −63 °F; 220 K (source[1]) |
| Boiling point | 171 to 173 °C; 340 to 343 °F; 444 to 446 K (source[1]) |
| Solubility in water | 41.3 g/L (25 ℃) |
| Hazards | |
| R-phrases | R10, R22, R23/24, R36/37/38 |
| S-phrases | S20, S27, S36/37/39, S45, S60 |
| NFPA 704 |
 3
1
0
|
| LD50 | 220 mg kg−1 (rat, oral) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
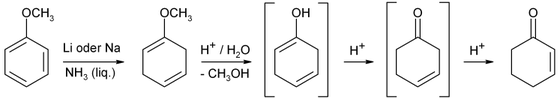
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances.[2] It is a clear colorless liquid in pure state but a commercially available product is mostly yellowish liquid.
Industrially, cyclohexenone is prepared from phenol by Birch reduction.[3]
Cyclohexenone is a ketone, or more precisely an enone. Common reactions include nucleophilic conjugate addition with organocopper reagents, Michael reactions and Robinson annulations.[4][5]
Property
It is soluble in many solvents, such as alcohols, ethers, haloalkanes, esters, and also is misible with polar aprotic solvents.
Cyclohexenone reacts both ketones and alkenes. It has an electron-poor carbon-carbon double bond as a typical representative of the α, β-unsaturated carbonyl compounds.
With strong bases, the positions 4 and 6 (the two CH2-groups of the carbonyl group and the C-C double bond adjacent) are deprotonated.
Synthesis
There are several different synthetic routes for the production of cyclohexenone. The reduction and acid hydrolysis of 3-ethoxy-2-cyclohexen-1-one is available for the laboratory scale. The precursor is accessible from resorcinol via 1,3-cyclohexanedione.
Anisole is also available. Cyclohexanone is obtained by Birch reduction followed by acid hydrolysis.
It can be obtained from cyclohexanone by α-bromination and elimination, or from 3-chloro cyclohexene by means of hydrolysis and oxidation.
Technically, 2-cyclohexen-1-one is produced by catalytic oxidation of cyclohexene, for example with hydrogen peroxide and vanadium catalysts. There are several patented process variants with different oxidizing agents or catalysts.
Reagent
It is a widely used building block in organic synthesis chemistry, as it offers many different ways of molecular framework extensions. For example, it is easy in a Michael addition with nucleophiles (such as enolates or silyl enol ethers) or will be implemented in a Diels-Alder reaction with electron-rich dienes. Further it reacts with organocopper compounds from 1,4-addition (Michael addition), or with Grignard reagents 1,2-addition, i.e., with attack of the nucleophile at the carbonyl carbon atom. It is used for example in multi-step synthesis in the construction of polycyclic natural products.
References
- ↑ 1.0 1.1 1.2 Cyclohexenone at Sigma-Aldrich
- ↑ Podraze, K.F. Org. Prep. Proced. Int., 1991, 23, p. 217.
- ↑ Organic Building Blocks of the Chemical Industry, Harry H. Szmant, ISBN 978-0-471-85545-3
- ↑ Michael G. Organ and Paul Anderson (1996). "Carbonyl and Conjugate Additions to Cyclohexenone: Experiments Illustrating Reagent Selectivity". Journal of Chemical Education 73 (12): 1193. doi:10.1021/ed073p1193.
- ↑ Tet. Lett. 34, 3881, (1993)