Cyclobutadieneiron tricarbonyl
| Cyclobutadieneiron tricarbonyl | ||
|---|---|---|
 | ||
| Identifiers | ||
| CAS number | 12078-17-0 | |
| Properties | ||
| Molecular formula | (C4H4)Fe(CO)3 | |
| Appearance | yellow solid | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Cyclobutadieneiron tricarbonyl or (C4H4)Fe(CO)3 is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state.[1]
Preparation and structure
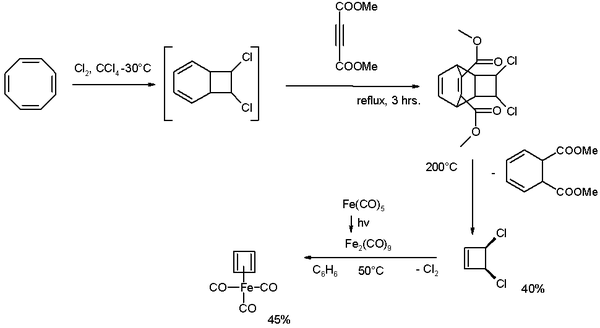
It was first prepared in 1965 by Rowland Pettit starting from cyclooctatetraene:[2][3][4]
Cyclooctatetraene is chlorinated to the [4.2.0]-bicyclic compound which reacts further with the alkyne dimethyl acetylenedicarboxylate in a Diels-Alder reaction followed by a reverse-DA reaction by pyrolysis at 200 °C releasing cis-dichlorocyclobutene. This compound reacts with di-iron nonacarbonyl (obtained from photolysis of iron pentacarbonyl) to give cyclobutadieneiron tricarbonyl.
The compound is a half sandwich complex. The C-C distances are 1.426 Â.P. D. Harvey, W. P. Schaefer, H. B. Gray, D. F. R. Gilson, I. S. Butler (1988). "Structure of tricarbonyl(η4-cyclobutadienyl)iron(0) at −45 °C". Inorg. Chem. 27 (1): 57–59. doi:10.1021/ic00274a013.
Properties
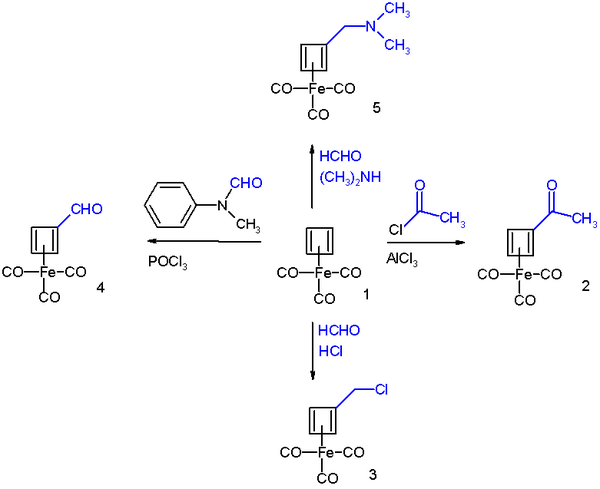
Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution:[5]
It undergoes Friedel-Crafts acylation with acetyl chloride and aluminium chloride to give the acyl derivative 2, with formaldehyde and hydrochloric acid to the chloromethyl derivative 3, in a Vilsmeier-Haack reaction with N-methylformanilide and phosphorus oxychloride to the formyl 4, and in a Mannich reaction to amine derivative 4.
The reaction mechanism is identical to that of EAS:
Related compounds
Several years before Petit's work, (C4Ph4)Fe(CO)3 had been prepared from the reaction of iron carbonyl and diphenylacetylene.[6]
References
- ↑ D. Seyferth "(Cyclobutadiene)iron Tricarbonyl - A Case of Theory before Experiment" Organometallics 2003, volume 22, 2-20.
- ↑ Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page
- ↑ Cis-dichlorocyclobutene , Organic Syntheses, Coll. Vol. 6, p.422 (1988); Vol. 50, p.36 (1970) Article.
- ↑ Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link
- ↑ Cyclobutadieneiron Tricarbonyl. A New Aromatic System J. D. Fitzpatrick, L. Watts, G. F. Emerson, R. Pettit J. Am. Chem. Soc.; 1965, vol. 87, 3254-3255 Abstract
- ↑ R. P. Dodge, V. Schomaker, "Crystal Structure of Tetraphenylcyclobutadiene Iron Tricarbonyl", Nature 1960, vol. 186, 798-799.doi:10.1038/186798b0