Cyanine
Cyanine is a non-systematic name of a synthetic dye family belonging to polymethine group. The word cyanin is from the English word “cyan", which conventionally means a shade of blue-green (close to "aqua") and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue".
Cyanines were and are still used in industry, and more recently in biotechnology (labeling, analysis). Cyanines have many uses as fluorescent dyes, particularly in biomedical imaging. Depending on the structure, they cover the spectrum from IR to UV. There are a large number reported in the literature.
Structure
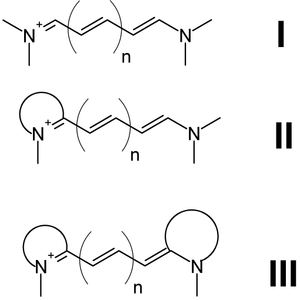
I = Streptocyanines,
II = Hemicyanines,
III = Closed cyanines
There are three types of cyanines:
- Streptocyanines or open chain cyanines:
- R2N+=CH[CH=CH]n-NR2 (I)
- Hemicyanines:
- Aryl=N+=CH[CH=CH]n-NR2 (II)
- Closed chain cyanines:[1]
- Aryl=N+=CH[CH=CH]n-N=Aryl (III)
where two nitrogens are joined by a polymethine chain.[2] Both nitrogens are each independently part of a heteroaromatic moiety, such as pyrrole, imidazole, thiazole, pyridine, quinoline, indole, benzothiazole, etc.
Cyanine dyes history, and use in industry
Cyanines were first synthesized over a century ago. They were originally used, and still are, to increase the sensitivity range of photographic emulsions, i.e., to increase the range of wavelengths which will form an image on the film, making the film panchromatic. Cyanines are also used in CD-R and DVD-R media. The ones used are mostly green or light blue in color, and are chemically unstable. This makes unstabilized cyanine discs unsuitable for archival CD and DVD use, as they can fade and become unreadable in a few years, however, recent cyanine discs contain stabilizers that slow down the deterioration significantly. These discs are often rated with an archival life of 75 years or more. The other dyes used in CD-Rs are phthalocyanine and azo.
Conventional Cyanines dyes used in biotechnology
| Probe | Ex (nm) | Em (nm) | MW | Quantum yield |
|---|---|---|---|---|
| Cy2 | 489 | 506 | 714 | QY 0.12 |
| Cy3 | (512);550 | 570;(615) | 767 | QY 0.15 |
| Cy3B | 558 | 572;(620) | 658 | QY 0.67 |
| Cy3.5 | 581 | 594;(640) | 1102 | QY 0.15 |
| Cy5 | (625);650 | 670 | 792 | QY 0.28 |
| Cy5.5 | 675 | 694 | 1128 | QY 0.28[3] |
| Cy7 | 743 | 767 | 818 | QY 0.28 |
Ex (nm): Excitation wavelength in nanometers
Em (nm): Emission wavelength in nanometers
MW: Molecular weight
QY: Quantum yield
Cy3 and Cy5
Cy 3 and Cy5 are the most popular cyanine dyes, used typically combined for 2 color detection. Cy3 dyes fluoresce yellow-green (~550 nm excitation, ~570 nm emission), while Cy5 is fluorescent in the red region (~650/670 nm) but absorbs in the orange region (~649 nm).[4]
They are usually synthesized with reactive groups on either one or both of the nitrogen side chains so that they can be chemically linked to either nucleic acids or protein molecules. Labeling is done for visualization and quantification purposes. They are used in a wide variety of biological applications including comparative genomic hybridization and in gene chips, which are used in transcriptomics. They are also used to label proteins and nucleic acid for various studies including proteomics and RNA localization.[5]

Nomenclature and structure
Standard chemical names specify exactly the chemical structure of the molecule. The Cy3 and Cy5 nomenclature was first proposed by Ernst, et al.[2] in 1989, and is non-standard, since it gives no hint of their chemical structures. In the original paper the number designated the count of the methines (as shown), and the side chains were unspecified. Thus various structures are designated Cy3 and Cy5 in the literature.
The R groups do not have to be identical. In the dyes as used they are short aliphatic chains one or both of which ends in a highly reactive moiety such as N-hydroxysuccinimide or maleimide.
Spectral characteristics
| Dye | Absorbance Max | Emission Max | Quantum yield in PBS buffer | Molecular weight (Da) |
|---|---|---|---|---|
| Cy3 | 550 nm | 570 nm | 0.04[6] | 766 |
| Cy5 | 649 nm | 670 nm | 0.28 | 792 |
The scanners actually use different laser emission wavelengths (typically 532 nm and 635 nm) and filter wavelengths (550-600 nm and 655-695 nm) to avoid background contamination. They are thus able to easily distinguish between two samples when one sample has been labeled with Cy3 and the other labeled with Cy5. They are also able to quantify the amount of labeling in either sample.
Cy dye alternatives
Many analogs of standard Cy 2 / 3 / 3.5 / 5 / 5.5 / 7 / 7.5 dyes were developed, using modification with moieties such as carboxyl, acetylmethoxy, sulfo,...: Alexa Fluor dyes, Dylight, FluoProbes dyes, Sulfo Cy dyes,[7] Seta dyes[8] and others can be used interchangeably with Cy dyes in most biochemical applications, with claimed improvements in solubility, fluorescence, or photostability.[9][10]
Cy5 ozone susceptibility
In 2003, researchers at Inpharmatics and Agilent reported in Analytical Chemistry that microarrays which used Cy5 were susceptible to intermittent data quality decrease caused by environmental ozone. Exposures to ozone levels above 5-10 ppb for 10–30 seconds were reported to decrease the reproducibility of Cy5 microarrays. Much higher levels of ozone (>100 ppb) were required to observe an effect in Cy3.[11] There are devices that claim to remove ambient ozone levels but they have not been 3rd party tested. Once such device is the Ozone Interceptor which claims to reduce ozone levels to less than 5 PPB.
Applications
Nucleic acid labeling
In microarray experiments DNA or RNA is labeled with either Cy3 or Cy5 that has been synthesized to carry an N-hydroxysuccinimidyl ester (NHS-ester) reactive group. Since NHS-esters react readily only with aliphatic amine groups, which nucleic acids lack, nucleotides have to be modified with aminoallyl groups. This is done through incorporating aminoallyl-modified nucleotides during synthesis reactions. A good ratio is a label every 60 bases such that the labels are not too close to each other, which would result in quenching effects.
Protein labeling
For protein labeling, Cy3 and Cy5 dyes sometimes bear maleimide reactive groups instead. The maleimide functionality allows conjugation of the fluorescent dye to the sulfhydryl group of cysteine residues. Cysteines can be added and removed from the protein domain of interest via PCR mutagenesis.
Cy5 is sensitive to the electronic environment it resides in. Changes in the conformation of the protein it is attached to will produce either enhancement or quenching of the emission. The rate of this change can be measured to determine enzyme kinetic parameters. The dyes can be used for similar purposes in FRET experiments.
Cy3 and Cy5 are used in proteomics experiments so that samples from two sources can be mixed and run together through the separation process.[12][13] This eliminates variations due to differing experimental conditions that are inevitable if the samples were run separately. These variations make it extremely difficult, if not impossible, to use computers to automate the acquisition of the data after the separation is complete. Using these dyes makes the automation trivial.
See also
References
- ↑ Johannes, H.H.: Cyanine: Direkte Funktionalisierung, Oligomerisierung, linear und nichtlinear optische Eigenschaften, Dissertation TU Braunschweig, 2000
- ↑ 2.0 2.1 Ernst LA, Gupta RK, Mujumdar RB, Waggoner AS (January 1989). "Cyanine dye labeling reagents for sulfhydryl groups". Cytometry 10 (1): 3–10. doi:10.1002/cyto.990100103. PMID 2917472.
- ↑ K. Umezawa,A. Matsui, Y. Nakamura, D. Citterio, K. Suzuke (2009). Chem. Eur. J. 15: 1096.
- ↑ Jackson ImmunoResearch. "Cyanine Dyes (Cy2, Cy3, and Cy5)". Retrieved 2008-10-31.
- ↑ Blower MD, Feric E, Weis K, Heald R (December 2007). "Genome-wide analysis demonstrates conserved localization of messenger RNAs to mitotic microtubules". The Journal of Cell Biology 179 (7): 1365–73. doi:10.1083/jcb.200705163. PMC 2373496. PMID 18166649.
- ↑ R.B. Mujumdar, L.A. Ernst, S.R. Mujumdar, C.J. Lewis, A.S. Waggoner, Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconj Chem 4, 105-111, 1993.
- ↑ Cyandye, LLC
- ↑ SETA BioMedicals
- ↑ FluoProbes488 comparison to FITC, Cyanine2
- ↑ FluoProbes547H comparison in Confocal Microscopy
- ↑ Fare TL, Coffey EM, Hongyue D, et al. Effects of Atmospheric Ozone on Microarray Data Quality. Analytical Chemistry. 2003;75:4672-4675.
- ↑ Unlü M, Morgan ME, Minden JS (October 1997). "Difference gel electrophoresis: a single gel method for detecting changes in protein extracts". Electrophoresis 18 (11): 2071–7. doi:10.1002/elps.1150181133. PMID 9420172.
- ↑ Ilya A. Osterman, Alexey V. Ustinov, Denis V. Evdokimov, Vladimir A. Korshun, Petr V. Sergiev, Marina V. Serebryakova, Irina A. Demina, Maria A. Galyamina, Vadim M. Govorun, Olga A. Dontsova (January 2013). "A nascent proteome study combining click chemistry with 2DE". PROTEOMICS 13 (1): 17–21. doi:10.1002/pmic.201200393. PMID 23161590.
External links
| Look up cyanine in Wiktionary, the free dictionary. |